

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50237347 CHEMBL4093477
SMILES: CCCN(CCCCNC(=O)c1cc2ccccc2[nH]1)[C@@H]1Cc2cccc3n(CCC)c(=O)n(C1)c23
InChI Key: InChIKey=HHDJZPNFXJLNJK-HSZRJFAPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50237347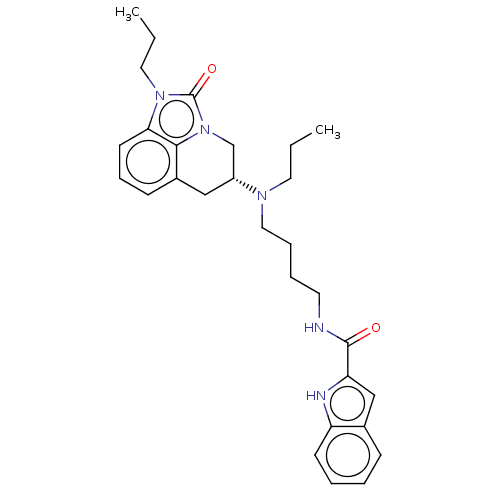 (CHEMBL4093477) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-(+)-7-OH-DPAT from human dopamine D3 receptor expressed in HEK293 cell membranes after 90 mins by micro beta scintillation c... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-(+)-7-OH-DPAT from human dopamine D2 receptor expressed in HEK293 cell membranes after 90 mins by micro beta scintillation c... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibitory constant against trypsin determined in Vitro | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 458 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor expressed in HEK293 cells coexpressing renilla luciferase-fused GalphaoA/GFP10-fused Ggamma2 by BRET a... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 338 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor expressed in HEK293 cells assessed as cAMP inhibition by BRET assay | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D2 receptor expressed in HEK293 cells coexpressing renilla luciferase-fused GalphaoA/GFP10-fused Ggamma2 assess... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at renilla luciferase-tagged human dopamine D2 receptor expressed in HEK293 cells coexpressing mVenus-fused beta-arrestin 2 asses... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D2 receptor expressed in HEK293 cells assessed as reduction in quinpirole-mediated cAMP inhibition preincubated... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonist activity at human dopamine D2 receptor expressed in HEK293 cells coexpressing renilla luciferase-fused Galphai1/GFP10-fused Ggamma2 by BRET a... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50237347 (CHEMBL4093477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 944 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonist activity at renilla luciferase-tagged human dopamine D2 receptor expressed in HEK293 cells coexpressing mVenus-fused beta-arrestin 2 assessed... | J Med Chem 60: 2890-2907 (2017) Article DOI: 10.1021/acs.jmedchem.6b01875 BindingDB Entry DOI: 10.7270/Q28P62SD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||