

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50237536 6-Shogaol::CHEBI:10138::SHOGAOL::Trans-6-Shogaol
SMILES: CCCCC\C=C\C(=O)CCc1ccc(O)c(OC)c1
InChI Key: InChIKey=OQWKEEOHDMUXEO-BQYQJAHWSA-N
Data: 14 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50237536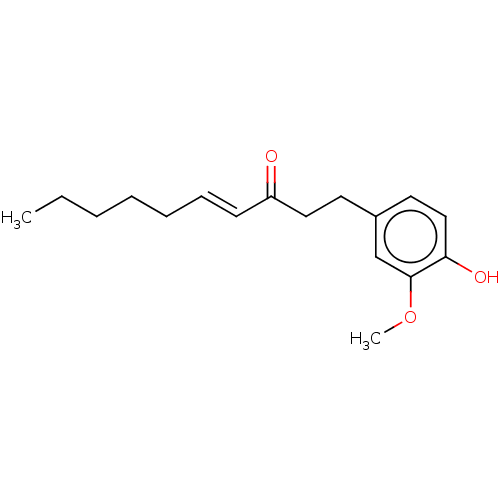 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by HPLC analysis | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2A6 in human liver microsomes using Coumarin as substrate preincubated for 10 mins followed by NADPH addition measured after 15 mins | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (hMC1R) | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate preincubated for 10 mins followed by NADPH addition measured after 20... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate by HPLC analysis | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 10 mins followed by NADPH addition measured after 10 mi... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 expressed in baculosomes using Vivid BOMR substrate red measured every 30 sec for 30 mins by fluorescence assa... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes using amodiaquin as substrate preincubated for 10 mins followed by NADPH addition measured after 10 mi... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC1R) | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 10 mins followed by NADPH addition measured after 5 mins | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate by HPLC analysis | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 10 mins followed by NADPH addition measured after 20 min... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 10 mins followed by NADPH addition measured after... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50237536 (6-Shogaol | CHEBI:10138 | SHOGAOL | Trans-6-Shogao...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 10 mins followed by NADPH addition measured after 10 ... | Bioorg Med Chem Lett 27: 1826-1830 (2017) Article DOI: 10.1016/j.bmcl.2017.02.047 BindingDB Entry DOI: 10.7270/Q2VD71Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||