

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50238062 CHEMBL4075081
SMILES: CCNC(=O)n1ncc2nccc(Nc3ccnc(c3)-c3cc(F)ccc3F)c12
InChI Key: InChIKey=VTZBBQMFTROBRY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50238062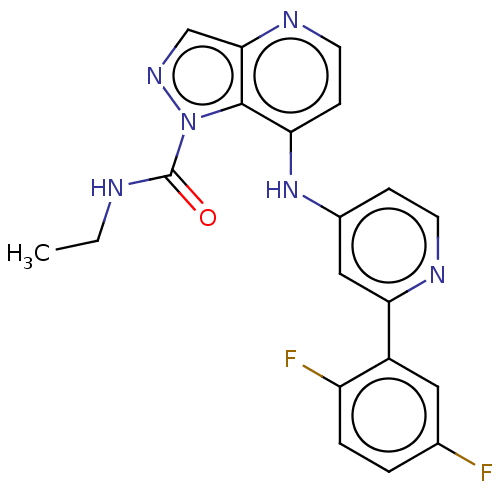 (CHEMBL4075081) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST tagged human ALK5 (200 to 503 residues) expressed in baculovirus expression system using FAM-NH2-KVLTQMGSPSI... | Bioorg Med Chem Lett 27: 1955-1961 (2017) Article DOI: 10.1016/j.bmcl.2017.03.026 BindingDB Entry DOI: 10.7270/Q2QJ7KKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50238062 (CHEMBL4075081) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Inhibition of ALK5 in human Calu6 cells assessed as reduction in Smad2/3 phosphorylation | Bioorg Med Chem Lett 27: 1955-1961 (2017) Article DOI: 10.1016/j.bmcl.2017.03.026 BindingDB Entry DOI: 10.7270/Q2QJ7KKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50238062 (CHEMBL4075081) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Takeda California Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Angiotensin I converting enzyme | Bioorg Med Chem Lett 27: 1955-1961 (2017) Article DOI: 10.1016/j.bmcl.2017.03.026 BindingDB Entry DOI: 10.7270/Q2QJ7KKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||