 Found 8 hits for monomerid = 50238242
Found 8 hits for monomerid = 50238242 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
A disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4)
(Homo sapiens (Human)) | BDBM50238242
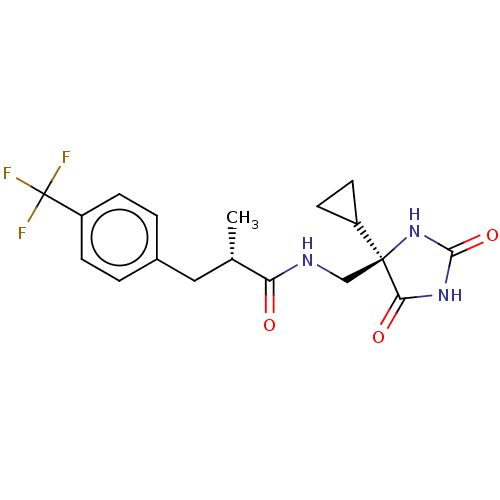
(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs by Alphascreen assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5)
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide as substrate after 3 hrs in the presence of 50% Lewis rat plasm... |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase 12
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Affinity towards cytoplasmic Thymidine kinase relative ot TdR; expressed as KM (TdR)/Ki |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase 14
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5)
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive-noncompetitive inhibition against rat mitochondrial thymidine kinase |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50238242

(CHEMBL4094455)Show SMILES C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)NC[C@]1(NC(=O)NC1=O)C1CC1 |r| Show InChI InChI=1S/C18H20F3N3O3/c1-10(8-11-2-4-13(5-3-11)18(19,20)21)14(25)22-9-17(12-6-7-12)15(26)23-16(27)24-17/h2-5,10,12H,6-9H2,1H3,(H,22,25)(H2,23,24,26,27)/t10-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 using Mca-PQGL-(3-[2, 4-dinitrophenyl]-L-2, 3-diaminopropionyl)-AR-OH as substrate after 2 to 4 hrs by fluorescence assay |
J Med Chem 60: 5933-5939 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00650
BindingDB Entry DOI: 10.7270/Q2Z321XM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data