

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50238580 CHEMBL4099451
SMILES: CSCCC[C@@H]1NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CO)NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N2
InChI Key: InChIKey=UMRUMCFCWHJXSF-MUJNREOGSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinic acetylcholine receptor alpha6/alpha3/beta4 (Rattus norvegicus-Rattus norvegicus (Rat)) | BDBM50238580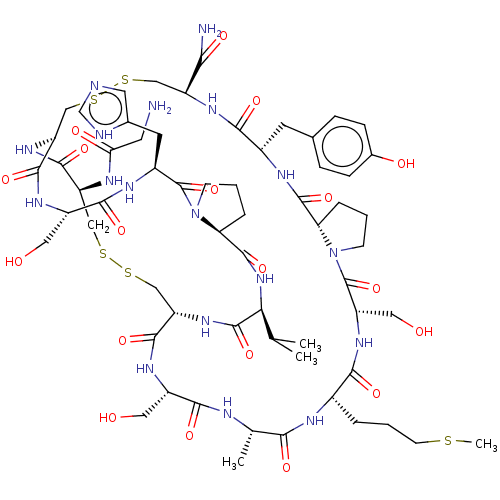 (CHEMBL4099451) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Antagonist activity at rat alpha6/alpha3beta4 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh induced channel current after 5... | J Med Chem 60: 5826-5833 (2017) Article DOI: 10.1021/acs.jmedchem.7b00546 BindingDB Entry DOI: 10.7270/Q28C9ZHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor Alpha-3/Beta-4 (Rattus norvegicus (Rat)) | BDBM50238580 (CHEMBL4099451) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University Curated by ChEMBL | Assay Description Antagonist activity at rat alpha3beta4 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh induced channel current after 5 mins a... | J Med Chem 60: 5826-5833 (2017) Article DOI: 10.1021/acs.jmedchem.7b00546 BindingDB Entry DOI: 10.7270/Q28C9ZHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||