 Found 11 hits for monomerid = 50238989
Found 11 hits for monomerid = 50238989 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM50238989
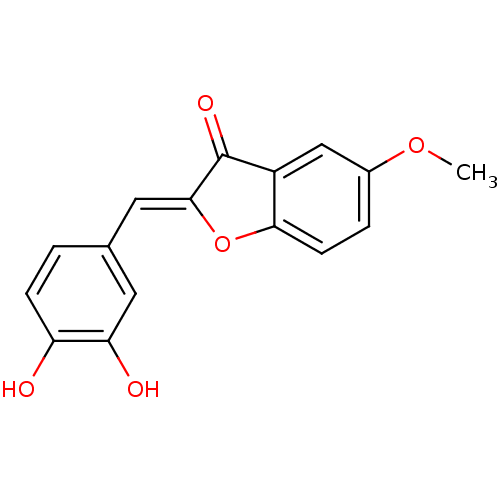
(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human AURORA-A |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human plasma renin |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | Reactome pathway
KEGG
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human BLK |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human TEK |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Cyclin-dependent-like kinase 5
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human CDK5 |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human ITK |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17B (STK17B)
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged DRAK2 (unknown origin) autophosphorylation after 2 hrs by ADP-glo assay |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17A
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | KEGG
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human DRAK1 |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Death-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
Reactome pathway
KEGG
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibitory constant for adenosine deaminase (ADA) |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Death-associated protein kinase 3
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human DAPK3 |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50238989

(CHEMBL4087337)Show InChI InChI=1S/C16H12O5/c1-20-10-3-5-14-11(8-10)16(19)15(21-14)7-9-2-4-12(17)13(18)6-9/h2-8,17-18H,1H3/b15-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China Normal University
Curated by ChEMBL
| Assay Description
Inhibitory activity against the thymidylate synthase in the Permeabilized L1210 cells |
Eur J Med Chem 130: 195-208 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.048
BindingDB Entry DOI: 10.7270/Q2XW4N3P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data