

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(Br)c2c(N)ncnn12
InChI Key: InChIKey=XXAVMCPRJUMLMZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50239748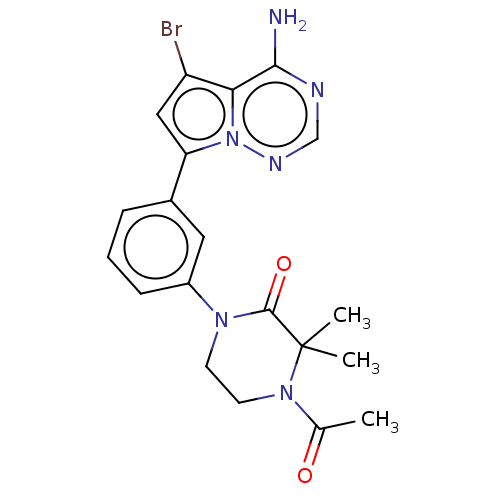 (CHEMBL4066316 | US10214537, Example 548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... | US Patent US10214537 (2019) BindingDB Entry DOI: 10.7270/Q2HH6NB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50239748 (CHEMBL4066316 | US10214537, Example 548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5... | J Med Chem 60: 5193-5208 (2017) Article DOI: 10.1021/acs.jmedchem.7b00618 BindingDB Entry DOI: 10.7270/Q2WW7KVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50239748 (CHEMBL4066316 | US10214537, Example 548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine | J Med Chem 60: 5193-5208 (2017) Article DOI: 10.1021/acs.jmedchem.7b00618 BindingDB Entry DOI: 10.7270/Q2WW7KVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||