

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50240485 18:0::C18:0::CH3-[CH2]16-COOH::CHEMBL46403::Octadecanoic acid::Octadecansaeure::Oktadekansaeure::Stearinsaeure::acide octadecanoique::acide stearique::n-octadecanoic acid::octadecoic acid::stearic acid
SMILES: CCCCCCCCCCCCCCCCCC(O)=O
InChI Key: InChIKey=QIQXTHQIDYTFRH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50240485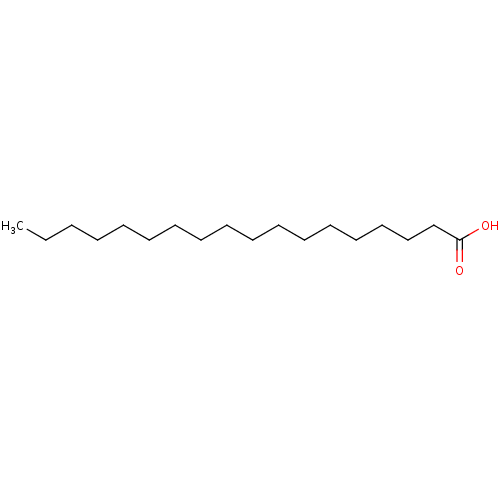 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and me... | Bioorg Med Chem Lett 28: 3315-3319 (2018) Article DOI: 10.1016/j.bmcl.2018.09.013 BindingDB Entry DOI: 10.7270/Q2SQ932J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and me... | Bioorg Med Chem Lett 28: 3315-3319 (2018) Article DOI: 10.1016/j.bmcl.2018.09.013 BindingDB Entry DOI: 10.7270/Q2SQ932J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 4 (FABP4) (Homo sapiens (Human)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against Adipocyte lipid binding protein | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured at 1... | Bioorg Med Chem Lett 28: 3315-3319 (2018) Article DOI: 10.1016/j.bmcl.2018.09.013 BindingDB Entry DOI: 10.7270/Q2SQ932J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by photometric method | J Nat Prod 83: 1598-1610 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (Cyclooxygenase-2) (Ovis aries (Sheep)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of sheep COX2-mediated prostaglandin biosynthesis using [1-14C]arachidonic acid | J Nat Prod 64: 745-9 (2001) BindingDB Entry DOI: 10.7270/Q24J0DVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]GW2433 from Homo sapiens (human) PPARdelta receptor by scintillation proximity assay | Citation and Details Article DOI: 10.1007/s00044-012-0285-6 BindingDB Entry DOI: 10.7270/Q2MG7SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]BRL49653 from Homo sapiens (human) PPARgamma receptor by scintillation proximity assay | Citation and Details Article DOI: 10.1007/s00044-012-0285-6 BindingDB Entry DOI: 10.7270/Q2MG7SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]GW2331 from Homo sapiens (human) PPARalpha receptor by scintillation proximity assay | Citation and Details Article DOI: 10.1007/s00044-012-0285-6 BindingDB Entry DOI: 10.7270/Q2MG7SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclooxygenase (Bos taurus) | BDBM50240485 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of bovine COX1-mediated prostaglandin biosynthesis using [1-14C]arachidonic acid | J Nat Prod 64: 745-9 (2001) BindingDB Entry DOI: 10.7270/Q24J0DVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||