

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50240895 2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone::2-Imidazol-1-yl-1-(4-nitro-phenyl)-ethanone::CHEMBL162549
SMILES: [O-][N+](=O)c1ccc(cc1)C(=O)Cn1ccnc1
InChI Key: InChIKey=SBPGWXSTCFNGPY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50240895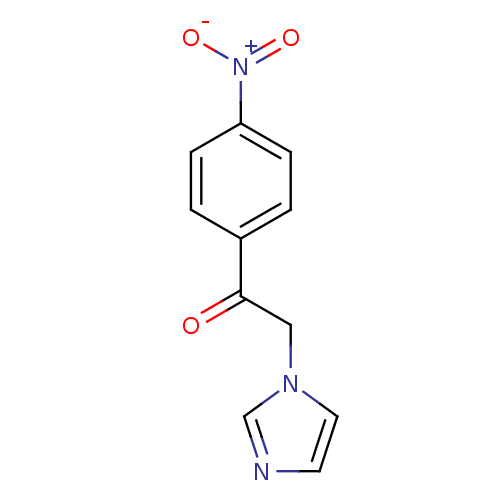 (2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Inhibition of nNOS (unknown origin) assessed as conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 16: 6193-206 (2008) Article DOI: 10.1016/j.bmc.2008.04.036 BindingDB Entry DOI: 10.7270/Q2611040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50240895 (2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of oxyhemoglobin to methemoglobin by UV spectro... | Eur J Med Chem 49: 118-26 (2012) Article DOI: 10.1016/j.ejmech.2012.01.002 BindingDB Entry DOI: 10.7270/Q2416XHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric-oxide synthase, endothelial (Mus musculus) | BDBM50240895 (2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania Curated by ChEMBL | Assay Description Inhibition of C57BL/6 mouse eNOS assessed as conversion of oxyhemoglobin to methemoglobin by UV spectrophotometer analysis | Eur J Med Chem 49: 118-26 (2012) Article DOI: 10.1016/j.ejmech.2012.01.002 BindingDB Entry DOI: 10.7270/Q2416XHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50240895 (2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human aromatase preincubated with NADP+ for 10 mins before substrate addition measured after 30 mins | Bioorg Med Chem 18: 5352-66 (2010) Article DOI: 10.1016/j.bmc.2010.05.042 BindingDB Entry DOI: 10.7270/Q2PK0H4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heme Oxygenase 1 (HO-1) (Rattus norvegicus (rat)) | BDBM50240895 (2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat spleen microsomal HO-1 assessed as bilirubin formation after 60 mins by spectrophotometric analysis | Bioorg Med Chem 21: 5145-53 (2013) Article DOI: 10.1016/j.bmc.2013.06.040 BindingDB Entry DOI: 10.7270/Q2FF3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heme Oxygenase 2 (HO-2) (Rattus norvegicus (rat)) | BDBM50240895 (2-(1H-Imidazol-1-yl)-1-(4-nitrophenyl)ethanone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat brain HO-2 assessed as bilirubin formation after 60 mins by spectrophotometric analysis | Bioorg Med Chem 21: 5145-53 (2013) Article DOI: 10.1016/j.bmc.2013.06.040 BindingDB Entry DOI: 10.7270/Q2FF3TSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||