

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50240958 CHEMBL4065350
SMILES: Cl.Oc1cccc(CCN(CCc2cccc(O)c2)CC2CCCCC2)c1
InChI Key: InChIKey=FINICKYVSVAWNA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240958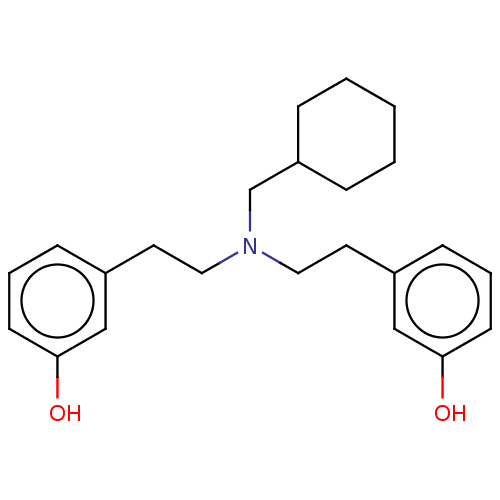 (CHEMBL4065350) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50240958 (CHEMBL4065350) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes after 60 mins by liquid scintillation counting method | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50240958 (CHEMBL4065350) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity towards sigma opioid receptor in guinea pig cerebral homogenate using [3H]DTG as radioligand | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50240958 (CHEMBL4065350) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of [3H]WIN-35428 binding to rat striatal membrane dopamine transporter | J Med Chem 60: 7579-7590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00981 BindingDB Entry DOI: 10.7270/Q21C202C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||