

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50241260 (3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-hydroxy-4,6a,6b,8a,11,12,14b-heptamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-icosahydropicene-4-carboxylic acid::CHEMBL267225::beta-boswellic acid::boswellic acid
SMILES: C[C@@H]1CC[C@]2(C)CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@@](C)([C@@H]5CC[C@@]34C)C(O)=O)[C@@H]2[C@H]1C
InChI Key: InChIKey=NBGQZFQREPIKMG-PONOSELZSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241260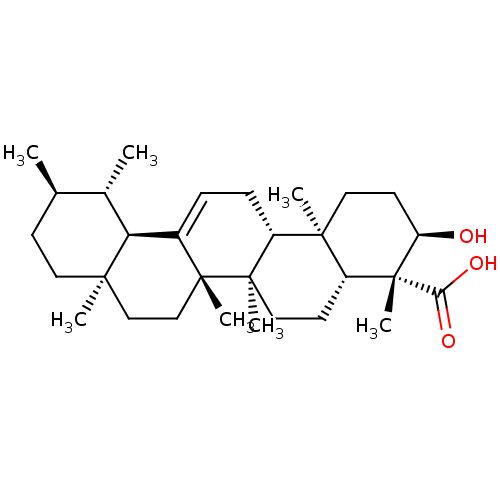 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) by pNPP assay | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of TCPTP (unknown origin) by pNPP assay | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of prolyl endopeptidase | J Nat Prod 68: 189-93 (2005) Article DOI: 10.1021/np040142x BindingDB Entry DOI: 10.7270/Q22V2H18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Laboratory of German Pharmacists Curated by ChEMBL | Assay Description Inhibition of mPGES1 | J Nat Prod 75: 1675-82 (2012) Article DOI: 10.1021/np300009w BindingDB Entry DOI: 10.7270/Q2P55PKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in insect cells assessed as decrease in production of 5-HPETE and 5-HETE using arachidonic acid as su... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 expressed in baculovirus infected sf21 cells assessed as decrease in PGE2 formation using arachidonic acid as su... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of ovine recombinant COX1 assessed as decrease in formation of PGE2 using arachidonic acid as substrate preincubated for 10 mins followed ... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of ovine recombinant COX1 assessed as decrease in formation of PGE2 using arachidonic acid as substrate preincubated for 10 mins followed ... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX expressed in insect cells assessed as decrease in production of 5-HPETE and 5-HETE using arachidonic acid as su... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 expressed in baculovirus infected sf21 cells assessed as decrease in PGE2 formation using arachidonic acid as su... | J Nat Prod 82: 3311-3320 (2019) Article DOI: 10.1021/acs.jnatprod.9b00538 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50241260 ((3R,4R,4aR,6aR,6bS,8aR,11R,12S,12aR,14aR,14bR)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abdul Wali Khan University Mardan Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cell microsomal membrane using pGH2 as substrate pretreated for 15 mins followed by substrate addition and measure... | Eur J Med Chem 153: 2-28 (2018) Article DOI: 10.1016/j.ejmech.2017.12.059 BindingDB Entry DOI: 10.7270/Q22R3V7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||