

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50241840 CHEMBL4072903::US10899756, Compound K
SMILES: CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C#N
InChI Key: InChIKey=CXMJWJJNVWXSJV-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241840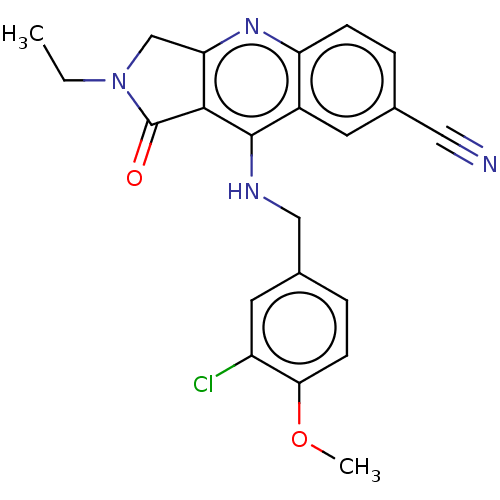 (CHEMBL4072903 | US10899756, Compound K) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of K+ stimulated gastric ATPase | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241840 (CHEMBL4072903 | US10899756, Compound K) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York US Patent | Assay Description A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... | US Patent US10899756 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50241840 (CHEMBL4072903 | US10899756, Compound K) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay | J Med Chem 60: 8858-8875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00979 BindingDB Entry DOI: 10.7270/Q2J67K3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||