

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50241937 (-)-dihydrocubebin::CHEMBL486597::dihydrocubebin
SMILES: OC[C@H](Cc1ccc2OCOc2c1)[C@H](CO)Cc1ccc2OCOc2c1
InChI Key: InChIKey=JKCVMTYNARDGET-HOTGVXAUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241937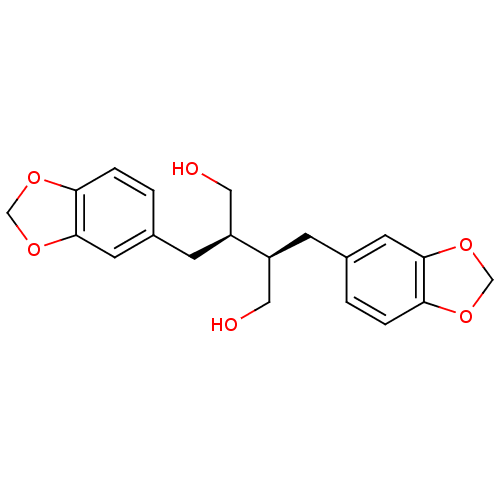 ((-)-dihydrocubebin | CHEMBL486597 | dihydrocubebin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycin | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50241937 ((-)-dihydrocubebin | CHEMBL486597 | dihydrocubebin) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human liver microsome CYP2D6 in assessed as [14C]formaldehyde formation | J Nat Prod 68: 64-8 (2005) Article DOI: 10.1021/np0401765 BindingDB Entry DOI: 10.7270/Q26M37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50241937 ((-)-dihydrocubebin | CHEMBL486597 | dihydrocubebin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human liver microsome CYP3A4 in assessed as [14C]formaldehyde formation | J Nat Prod 68: 64-8 (2005) Article DOI: 10.1021/np0401765 BindingDB Entry DOI: 10.7270/Q26M37Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||