

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50242173 (2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4H-chromen-7-yloxy)-3,4,5-trihydroxy-tetrahydro-2H-pyran-2-carboxylic acid::Baicalin (9)::CHEMBL485818::baicalin
SMILES: O[C@H]1[C@H](Oc2cc3oc(cc(=O)c3c(O)c2O)-c2ccccc2)O[C@@H]([C@@H](O)[C@@H]1O)C(O)=O
InChI Key: InChIKey=IKIIZLYTISPENI-ZFORQUDYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canalicular multispecific organic anion transporter 1 (Rattus norvegicus) | BDBM50242173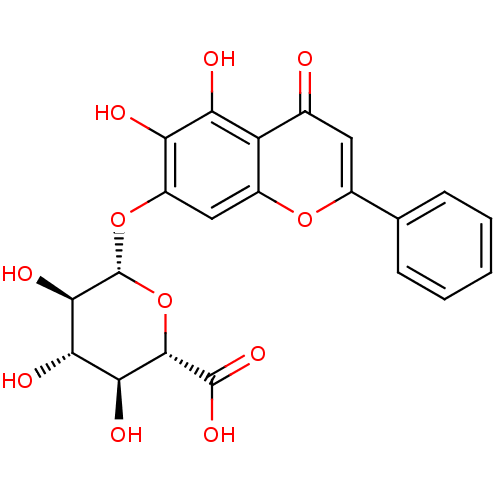 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of DNP-SG uptake in bile canalicular membrane vesicles from SD rat | Drug Metab Pharmacokinet 17: 23-33 (2002) Article DOI: 10.2133/dmpk.17.23 BindingDB Entry DOI: 10.7270/Q22B91VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Tripura University, Suryamaninagar 799022, Tripura, India. Electronic address: dindabtu@gmail.com. Curated by ChEMBL | Assay Description Mixed-type inhibition of CYP1A2 in pooled human liver microsomes using varying levels of phenacetin as substrate pretreated for 5 mins followed by su... | Eur J Med Chem 131: 68-80 (2017) Article DOI: 10.1016/j.ejmech.2017.03.004 BindingDB Entry DOI: 10.7270/Q2R49T6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells | J Nat Prod 66: 535-7 (2003) Article DOI: 10.1021/np0205102 BindingDB Entry DOI: 10.7270/Q2CZ36WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recerca Biomèdica de Barcelona Curated by ChEMBL | Assay Description Inhibition of human brain prolyl oligopeptidase expressed in Escherichia coli | Bioorg Med Chem 16: 7516-24 (2008) Article DOI: 10.1016/j.bmc.2008.04.067 BindingDB Entry DOI: 10.7270/Q2ZC82NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ehime University Curated by ChEMBL | Assay Description Inhibition of trypsin-induced elevation in PAI1 production in HUVEC by ELISA | J Nat Prod 60: 598-601 (1997) Article DOI: 10.1021/np970035l BindingDB Entry DOI: 10.7270/Q20C4ZK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant POP | J Med Chem 53: 3423-38 (2010) Article DOI: 10.1021/jm901104g BindingDB Entry DOI: 10.7270/Q261119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) | Bioorg Med Chem 27: 370-374 (2019) Article DOI: 10.1016/j.bmc.2018.12.013 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Cdk1/cyclin B | Bioorg Med Chem 16: 7128-33 (2008) Article DOI: 10.1016/j.bmc.2008.06.055 BindingDB Entry DOI: 10.7270/Q2JQ11XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University | Assay Description The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The... | Chem Biol Drug Des 85: 574-85 (2015) Article DOI: 10.1111/cbdd.12445 BindingDB Entry DOI: 10.7270/Q2M61J08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50242173 ((2S,3S,4S,5R,6S)-6-(5,6-dihydroxy-4-oxo-2-phenyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recerca Biomèdica de Barcelona Curated by ChEMBL | Assay Description Inhibition of DPP4 (unknown origin) | Bioorg Med Chem 16: 7516-24 (2008) Article DOI: 10.1016/j.bmc.2008.04.067 BindingDB Entry DOI: 10.7270/Q2ZC82NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||