

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50243842 CHEMBL4066404::US10966980, Example 80
SMILES: CCCCN(C)S(=O)(=O)C[C@H]1C[C@H](C1)N(C)c1ncnc2[nH]ccc12
InChI Key: InChIKey=WGAJDNFRQOWUQD-OKILXGFUSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50243842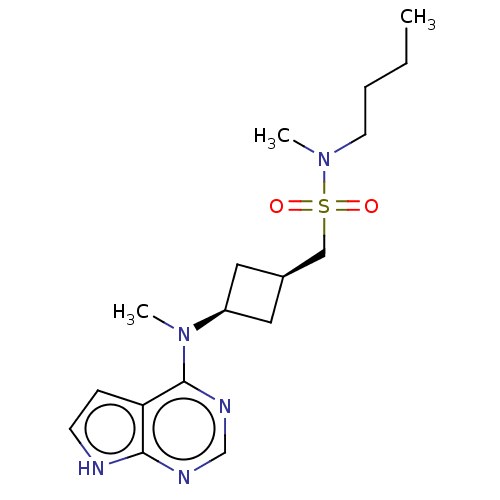 (CHEMBL4066404 | US10966980, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK1 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay | J Med Chem 61: 1130-1152 (2018) Article DOI: 10.1021/acs.jmedchem.7b01598 BindingDB Entry DOI: 10.7270/Q2D79DTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human TYK2 using 5'FAM-KKSRGDYMTMQID as substrate in presence of 1 mM ATP by mobility shift assay | J Med Chem 61: 1130-1152 (2018) Article DOI: 10.1021/acs.jmedchem.7b01598 BindingDB Entry DOI: 10.7270/Q2D79DTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK1/TYK2 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]GR-65630 binding to 5-hydroxytryptamine 3 receptor | J Med Chem 61: 1130-1152 (2018) Article DOI: 10.1021/acs.jmedchem.7b01598 BindingDB Entry DOI: 10.7270/Q2D79DTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of JAK2 in CD34+ human whole blood assessed as reduction in EOP induced STAT5 phosphorylation preincubated for 45 mins followed by EOP add... | J Med Chem 61: 1130-1152 (2018) Article DOI: 10.1021/acs.jmedchem.7b01598 BindingDB Entry DOI: 10.7270/Q2D79DTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 886 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Test article and assay controls were added to a 384-well plate. Reaction mixtures contained 20 mM HEPES, pH 7.4, 10 mM magnesium chloride, 0.01% bovi... | US Patent US10966980 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50243842 (CHEMBL4066404 | US10966980, Example 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 888 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human JAK2 using FITC-KGGEEEEYFELVKK as substrate in presence of 1 mM ATP by mobility shift assay | J Med Chem 61: 1130-1152 (2018) Article DOI: 10.1021/acs.jmedchem.7b01598 BindingDB Entry DOI: 10.7270/Q2D79DTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||