

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50244276 2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benzo[d]imidazole-5-carboxamide::CHEMBL471980
SMILES: CCOc1ccc(Cc2nc3cc(ccc3n2CCC(C)C)C(=O)N(CC)CC)cc1
InChI Key: InChIKey=RLLSCGRLMPJHDQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276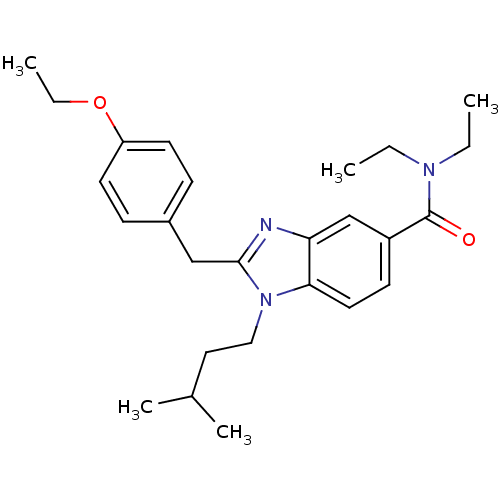 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor | Bioorg Med Chem Lett 18: 3695-700 (2008) Article DOI: 10.1016/j.bmcl.2008.05.073 BindingDB Entry DOI: 10.7270/Q2J38SBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Binding affinity in human CB2R expressed in human HEK cells incubated for 3 hrs by microbeta scintillation counting | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human recombinant cannabinoid CB2 receptor expressed in human HEK cells by radioligand binding assay | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor | Bioorg Med Chem Lett 18: 3695-700 (2008) Article DOI: 10.1016/j.bmcl.2008.05.073 BindingDB Entry DOI: 10.7270/Q2J38SBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montréal Curated by ChEMBL | Assay Description Agonist activity at cloned human CB2 receptor in Sf9 cells assessed as stimulation of [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3695-700 (2008) Article DOI: 10.1016/j.bmcl.2008.05.073 BindingDB Entry DOI: 10.7270/Q2J38SBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor expressed in Sf9 cells coexpressing Galpha i2 assessed as Galpha GTPase activity using [gamma-33P]GTP by scint... | Bioorg Med Chem 22: 3938-46 (2014) Article DOI: 10.1016/j.bmc.2014.06.008 BindingDB Entry DOI: 10.7270/Q2862J3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in Sf9 cells coexpressing Galpha i2 assessed as Galpha GTPase activity using [gamma-33P]GTP by scint... | Bioorg Med Chem 22: 3938-46 (2014) Article DOI: 10.1016/j.bmc.2014.06.008 BindingDB Entry DOI: 10.7270/Q2862J3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of equine BChE using BTC iodide as substrate preincubated for 4.5 mins followed by substrate addition measured after 2.5 mins by Ellman's ... | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||