

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50244296 (-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]methyl}-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-ol::CHEMBL472168::trans-(+)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-ol
SMILES: Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC1
InChI Key: InChIKey=RWZHKEZRFILBTQ-GCJKJVERSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244296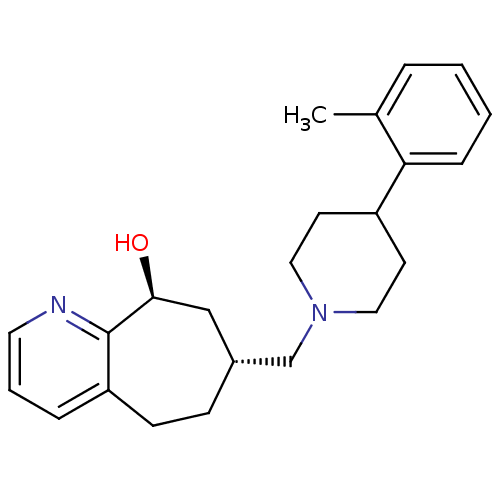 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244296 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Agonist activity at human ORL1 expressed in CHO cells by GTPgammaS binding assay relative to nociceptin | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50244296 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]MK499 from human ERG K+ channel expressed in HEK293 cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50244296 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]MK499 from human ERG K+ channel expressed in HEK293 cells | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50244296 ((-)-(7R,9S)-7-{[4-(2-Methylphenyl)piperidin-1-yl]m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 expressed in CHO cells assessed as effect on nociceptin-induced GTPgammaS binding | J Med Chem 51: 4021-9 (2008) Article DOI: 10.1021/jm701590h BindingDB Entry DOI: 10.7270/Q2KW5FTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||