

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1cc(C)c(CN2CCc3c(Cl)cc(C(CO)C4CCOCC4)c(Cl)c3C2=O)c(=O)[nH]1
InChI Key: InChIKey=GJPUEPDRSRFYRD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246944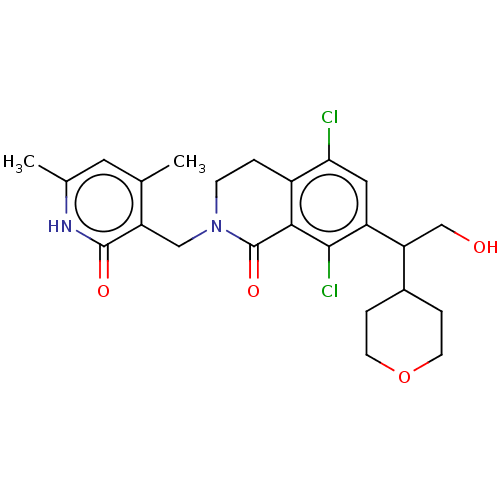 (CHEMBL4080606 | US10570121, Example 38) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Curated by ChEMBL | Assay Description Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA | J Med Chem 61: 650-665 (2018) Article DOI: 10.1021/acs.jmedchem.7b01375 BindingDB Entry DOI: 10.7270/Q2X069G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Curated by ChEMBL | Assay Description Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27me3 level after 72 hrs by ELISA | J Med Chem 61: 650-665 (2018) Article DOI: 10.1021/acs.jmedchem.7b01375 BindingDB Entry DOI: 10.7270/Q2X069G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [2-746,Y641N] (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [2-746,Y641N] (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 [2-746,Y641N] (Homo sapiens (Human)) | BDBM50246944 (CHEMBL4080606 | US10570121, Example 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description A. Compound preparation1. Prepare 10 mM stock solutions in 100% DMSO from solid material2. Serial dilute 10 mM compound stocks either 2 or 3-fold in ... | US Patent US10570121 (2020) BindingDB Entry DOI: 10.7270/Q28W3GQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||