

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50247743 CHEMBL504206::[(1R,2R,6R,10S,11R,15S,17R)-13-benzyl-6-hydroxy-4,17-dimethyl-5-oxo-15-(prop-1-en-2-yl)-12,14,18-trioxapentacyclo[11.4.1.0^{1,10}.0^{2,6}.0^{11,15}]octadeca-3,8-dien-8-yl]methyl 2-(4-methanesulfonamidophenyl)acetate
SMILES: C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccc(NS(C)(=O)=O)cc4)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C
InChI Key: InChIKey=GYTMPLYSWIONAO-ARGYEFFCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanilloid receptor (Rattus norvegicus (rat)) | BDBM50247743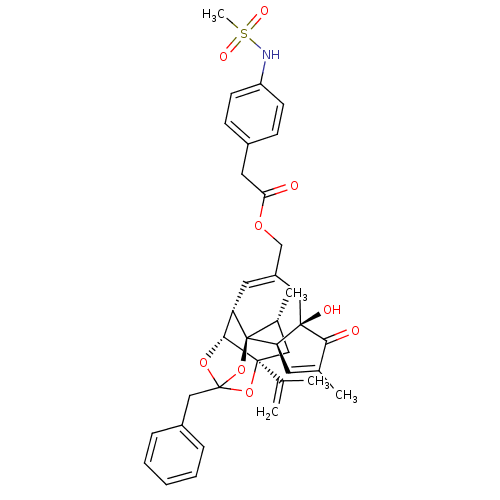 (CHEMBL504206 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vanilloid receptor (Rattus norvegicus (rat)) | BDBM50247743 (CHEMBL504206 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Agonist activity at rat TRPV1 receptor expressed in CHO cells assessed as calcium uptake | Bioorg Med Chem 17: 690-8 (2009) Article DOI: 10.1016/j.bmc.2008.11.085 BindingDB Entry DOI: 10.7270/Q2CJ8FCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||