

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1[nH]n(-c2ccc(C)c(C)c2)c(=O)c1N=Nc1cccc(-c2cccc(c2)C(O)=O)c1O
InChI Key: InChIKey=SVOQIEJWJCQGDQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute carrier organic anion transporter family member 2B1 (Homo sapiens (Human)) | BDBM50248106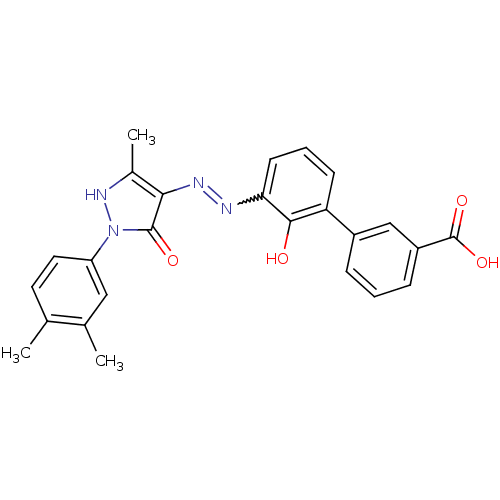 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of OATP2B1-mediated [3H]estrone-3-sulfate uptake in human OATP2B1 expressing HEK293/PDZK1 cells by scintillation counting | Drug Metab Dispos 39: 1088-96 (2011) Article DOI: 10.1124/dmd.110.037960 BindingDB Entry DOI: 10.7270/Q2RB76BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B1 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of OATP1B1-mediated [3H]estrone-3-sulfate uptake in human OATP1B1 expressing HEK293/PDZK1 cells by scintillation counting | Drug Metab Dispos 39: 1088-96 (2011) Article DOI: 10.1124/dmd.110.037960 BindingDB Entry DOI: 10.7270/Q2RB76BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of OATP1B3-mediated [3H]estradiol 17beta-glucuronide uptake in human OATP1B3 expressing HEK293/PDZK1 cells by scintillation counting | Drug Metab Dispos 39: 1088-96 (2011) Article DOI: 10.1124/dmd.110.037960 BindingDB Entry DOI: 10.7270/Q2RB76BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 1 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of OCT1-mediated [14C]tetraethylammonium uptake in human OCT1 expressing HEK293/PDZK1 cells by scintillation counting | Drug Metab Dispos 39: 1088-96 (2011) Article DOI: 10.1124/dmd.110.037960 BindingDB Entry DOI: 10.7270/Q2RB76BH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of fast sodium current (INa) in HEK293 cells transfected with human Nav1.5 measured using IonWorks Quattro automated patch clamp platform | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombopoietin receptor (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Agonist activity at human thrombopoietin receptor expressed in mouse Ba/F3 cells by kinase activation based reporter gene assay | Bioorg Med Chem Lett 18: 5259-62 (2008) Article DOI: 10.1016/j.bmcl.2008.08.077 BindingDB Entry DOI: 10.7270/Q2ZP45ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GSC2 (Saccharomyces cerevisiae) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Medicine of University of Electronic Science and Technology of China Curated by ChEMBL | Assay Description Inhibition of human ERG channel tail current | Bioorg Med Chem 24: 1419-30 (2016) Article DOI: 10.1016/j.bmc.2016.02.030 BindingDB Entry DOI: 10.7270/Q22N5444 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thrombopoietin receptor (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Agonist activity at human thrombopoietin receptor in Ba/F3 cells assessed as activation of Stat5 response element-driven reporter gene expression | Bioorg Med Chem Lett 18: 5255-8 (2008) Article DOI: 10.1016/j.bmcl.2008.08.068 BindingDB Entry DOI: 10.7270/Q2HH6M05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Korea | Assay Description Ten-point DRCs were generated for each drug. Vero cells were seeded at 1.2 × 104 cells per well in DMEM, supplemented with 2% FBS and 1× ... | Antimicrob Agents Chemother 64: (2020) Article DOI: 10.1128/AAC.00819-20 BindingDB Entry DOI: 10.7270/Q22N54QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The 3CLpro enzyme assay was developed in 384-well black, medium binding microplates (Greiner Bio-One, Monroe, NC, USA) with a total volume of 20 _... | bioRxiv (2020) Article DOI: 10.1101/2020.07.17.207019 BindingDB Entry DOI: 10.7270/Q20G3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description SARS-CoV-2 CPE assay was conducted at Southern Research Institute (Birmingham, AL) as described in previous reports30, 31. In brief, high ACE2 expres... | bioRxiv (2020) Article DOI: 10.1101/2020.07.17.207019 BindingDB Entry DOI: 10.7270/Q20G3NJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This is a review article. Please point to the original journal. | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00409 BindingDB Entry DOI: 10.7270/Q2J1069F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of rapid delayed inward rectifying potassium current (IKr) in Chinese hamster ovary (CHO) cells stable expressing hERG measured using IonW... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily D member 3 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of fast sodium current (INa) in HEK293 cells transfected with human Nav1.5 measured using IonWorks Quattro automated patch clamp platform | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Broad substrate specificity ATP-binding cassette transporter ABCG2 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114346 BindingDB Entry DOI: 10.7270/Q2PZ5DT3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of slow delayed inward rectifying potassium current (Iks) in Chinese Hamster Ovary (CHO) cells expressing hKvLQT1/hminK measured using Ion... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50248106 (CHEMBL461101 | ELTROMBOPAG | Eltrombopag olamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of transient outward potassium current (Ito) current in Chinese Hamster Ovary (CHO) K1 cells expressing human Kv4.3 measured using IonWork... | J Pharmacol Toxicol Methods 70: 246-54 (2014) Article DOI: 10.1016/j.vascn.2014.07.002 BindingDB Entry DOI: 10.7270/Q2J104W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||