

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50249150 8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2.0^{2,7}.0^{9,14}]hexadeca-2,4,6,9(14),10,12-hexaene-15-carboxamide::CHEMBL474189
SMILES: CC1(CC2(C)c3ccccc3C1c1ccccc21)C(=O)Nc1nccs1
InChI Key: InChIKey=RKZJWUOBFCJJTR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150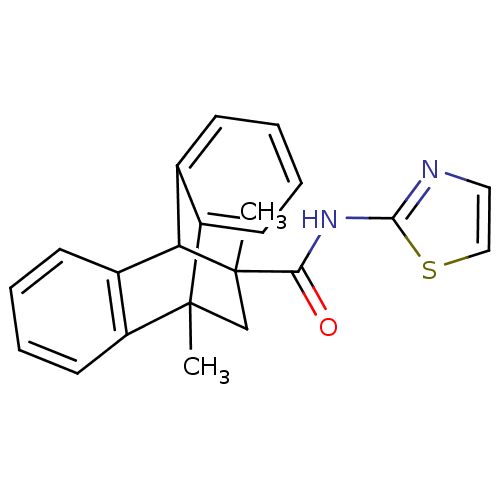 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of FITC-dexamethasone from human recombinant glucocorticoid receptor alpha by fluorescence polarization assay | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from glucocorticoid receptor by fluorescence polarization assay | J Med Chem 54: 7318-33 (2011) Article DOI: 10.1021/jm200879j BindingDB Entry DOI: 10.7270/Q2W0969M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of radioligand from progesterone receptor by fluorescence polarization assay | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of FITC-dexamethasone from human recombinant glucocorticoid receptor alpha by fluorescence polarization assay | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of radioligand from androgen receptor by fluorescence polarization assay | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of radioligand from estrogen receptor alpha by fluorescence polarization assay | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transrepression activity at GR in IL-1-beta-stimulated human A549 cells assessed as inhibition of NF-kappaB-dependent E-selectin transcription by luc... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transrepression activity at GR in IL-1-beta-stimulated human A549 cells assessed as inhibition of NF-kappaB-dependent E-selectin transcription by luc... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 121 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at GR ligand binding domain expressed in human NP1 cells assessed as glucocorticoid response element transactivation by GAL4 lucifer... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at GR ligand binding domain expressed in human NP1 cells assessed as glucocorticoid response element transactivation by GAL4 lucifer... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at GR ligand binding domain expressed in human NP1 cells assessed as glucocorticoid response element transactivation by GAL4 lucifer... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transrepression activity at GR in IL-1-beta-stimulated human A549 cells assessed as inhibition of NF-kappaB-dependent E-selectin transcription by luc... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transrepression activity at GR in PMA-stimulated human A549 cells assessed as inhibition of AP1 response element-induced luciferase reporter gene act... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transrepression activity at GR in PMA-stimulated human A549 cells assessed as inhibition of AP1 response element-induced luciferase reporter gene act... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14.7 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transrepression activity at GR in PMA-stimulated human A549 cells assessed as inhibition of AP1 response element-induced luciferase reporter gene act... | Bioorg Med Chem Lett 19: 2139-43 (2009) Article DOI: 10.1016/j.bmcl.2009.03.006 BindingDB Entry DOI: 10.7270/Q2XW4KRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor alpha in phorbol myristate acetate-stimulated human A549 cells assessed as inhibition of AP1 resp... | J Med Chem 54: 7318-33 (2011) Article DOI: 10.1021/jm200879j BindingDB Entry DOI: 10.7270/Q2W0969M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor alpha in IL-1beta-stimulated human A549 cells assessed as inhibition of NFkappaB-dependent E-sele... | J Med Chem 54: 7318-33 (2011) Article DOI: 10.1021/jm200879j BindingDB Entry DOI: 10.7270/Q2W0969M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50249150 (8,15-dimethyl-N-(1,3-thiazol-2-yl)tetracyclo[6.6.2...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transactivation activity at GR-alpha in human NP1 Hela cells assessed as induction of GAL4-DBD after 20 hrs by luciferase reporter gene assay | J Med Chem 54: 7318-33 (2011) Article DOI: 10.1021/jm200879j BindingDB Entry DOI: 10.7270/Q2W0969M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||