

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@@]12Cc3ccc(O)c4O[C@@]5(C)CCC[C@]1(OC)[C@@]5(CCN2C)c34
InChI Key: InChIKey=IWNLIUUGJPKDCB-GRGSLBFTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50250451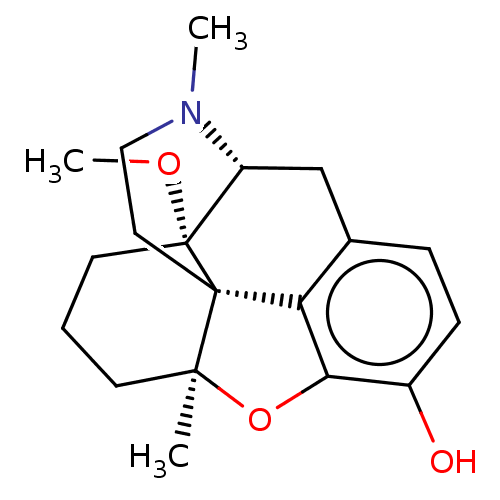 (CHEMBL4088589) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from recombinant human MOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting | J Med Chem 60: 9407-9412 (2017) Article DOI: 10.1021/acs.jmedchem.7b01363 BindingDB Entry DOI: 10.7270/Q2PZ5C72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50250451 (CHEMBL4088589) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]-HS665 from recombinant human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting | J Med Chem 60: 9407-9412 (2017) Article DOI: 10.1021/acs.jmedchem.7b01363 BindingDB Entry DOI: 10.7270/Q2PZ5C72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50250451 (CHEMBL4088589) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from recombinant human DOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting | J Med Chem 60: 9407-9412 (2017) Article DOI: 10.1021/acs.jmedchem.7b01363 BindingDB Entry DOI: 10.7270/Q2PZ5C72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50250451 (CHEMBL4088589) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Agonist activity at recombinant human MOR expressed in CHO cell membranes after 60 mins by [35S]GTPgammaS binding assay | J Med Chem 60: 9407-9412 (2017) Article DOI: 10.1021/acs.jmedchem.7b01363 BindingDB Entry DOI: 10.7270/Q2PZ5C72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||