

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50251013 2,4,2',4'-tetrahydroxy-3'-prenylchalcone::CHEMBL465880::morachalcone A
SMILES: [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])cc2-[#8])c1-[#8]
InChI Key: InChIKey=NXBYIJSAISXPKJ-WEVVVXLNSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50251013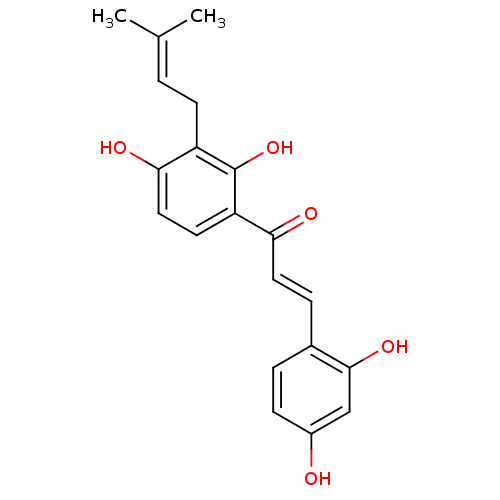 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of aromatase from human placental microsomes | J Nat Prod 64: 1286-93 (2001) BindingDB Entry DOI: 10.7270/Q2RB74C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic lipase (Sus scrofa (Pig)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenylbutyrate as substrate assessed as formation of p-nitrophenol preincubated for 15 mins foll... | Bioorg Med Chem Lett 25: 2269-74 (2015) Article DOI: 10.1016/j.bmcl.2015.04.045 BindingDB Entry DOI: 10.7270/Q2GF0W7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||