 Found 11 hits for monomerid = 50252103
Found 11 hits for monomerid = 50252103 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate kainate
(RABBIT) | BDBM50252103
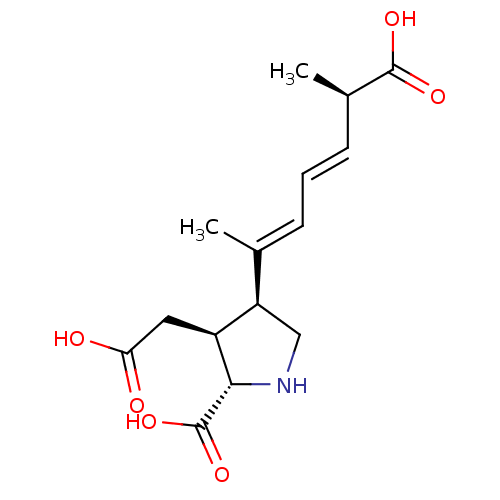
((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 1483-8 (1997)
Article DOI: 10.1016/s0028-3908(97)00161-5
BindingDB Entry DOI: 10.7270/Q2C827T6 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(RAT) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Blaise Pascal
Curated by ChEMBL
| Assay Description
Displacement of [3H]SYM2081 from rat recombinant iGluR5 |
J Med Chem 51: 4093-103 (2008)
Article DOI: 10.1021/jm800092x
BindingDB Entry DOI: 10.7270/Q20V8DP6 |
More data for this
Ligand-Target Pair | |
GRIA3
(RAT) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate
(Rattus norvegicus) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Blaise Pascal
Curated by ChEMBL
| Assay Description
Displacement of [3H]SYM2081 from rat recombinant iGluR7 |
J Med Chem 51: 4093-103 (2008)
Article DOI: 10.1021/jm800092x
BindingDB Entry DOI: 10.7270/Q20V8DP6 |
More data for this
Ligand-Target Pair | |
Ionotropic glutamate receptor kainate 2/5
(Rattus norvegicus) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Blaise Pascal
Curated by ChEMBL
| Assay Description
Displacement of [3H]SYM2081 from rat recombinant iGluR6 |
J Med Chem 51: 4093-103 (2008)
Article DOI: 10.1021/jm800092x
BindingDB Entry DOI: 10.7270/Q20V8DP6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
GRIK4
(Homo sapiens (Human)) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allelix Biopharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
J Neurochem 62: 1-9 (1994)
Article DOI: 10.1046/j.1471-4159.1994.62010001.x
BindingDB Entry DOI: 10.7270/Q2RJ4H0P |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic kainate 4
(Rattus norvegicus) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Blaise Pascal
Curated by ChEMBL
| Assay Description
Binding affinity to rat cloned KA1 receptor |
J Med Chem 51: 4093-103 (2008)
Article DOI: 10.1021/jm800092x
BindingDB Entry DOI: 10.7270/Q20V8DP6 |
More data for this
Ligand-Target Pair | |
GRIA3
(RAT) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 927 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 650-8 (2001)
BindingDB Entry DOI: 10.7270/Q2M0440C |
More data for this
Ligand-Target Pair | |
GRIA3
(Homo sapiens (Human)) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
GRIA3
(RAT) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
GRIA3
(Homo sapiens (Human)) | BDBM50252103

((2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-MET...)Show SMILES C[C@H](\C=C\C=C(/C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4+/t9-,10+,11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data