

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50252865 (+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fluoro-4-hydroxymethylpyrrolidin-3-ol::(3S,4S)-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fluoro-4-hydroxymethyl pyrrolidin-3-ol::CHEMBL498940::Immucillins, 1
SMILES: OC[C@@]1(F)CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O
InChI Key: InChIKey=VFUSATHXTREUCA-UFBFGSQYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865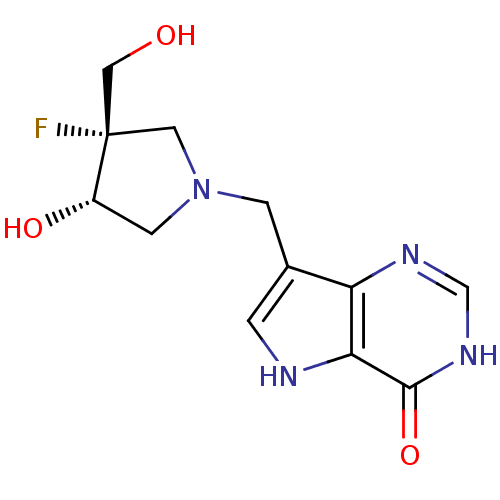 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine Nucleoside Phosphorylase (PNP) (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine Nucleoside Phosphorylase (PNP) (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine Nucleoside Phosphorylase (PNP) (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine Nucleoside Phosphorylase (PNP) (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell (A) | Syringe (B) | Cell Links | Syringe Links | Cell + Syr Links | ΔG° kcal/mole | -TΔS° kcal/mole | ΔH° kcal/mole | log K | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | DrugBank GoogleScholar KEGG PDB | PC cid PC sid | -60.2 | n/a | n/a | n/a | 7.40 | 25 | |
Albert Einstein College of Medicine | Biochemistry 48: 5226-38 (2009) | |||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | DrugBank GoogleScholar KEGG PDB | PC cid PC sid | n/a | n/a | -22.7 | n/a | 7.40 | 27 | |
Albert Einstein College of Medicine | Biochemistry 48: 5226-38 (2009) | |||||||||