

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50254184 CHEMBL4082366
SMILES: CC(C)(O)CC(=O)N1CCC(CC1)c1ccc(NC(=O)N2Cc3ccccc3C2)cc1
InChI Key: InChIKey=GBPFJQPCQMDAAN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254184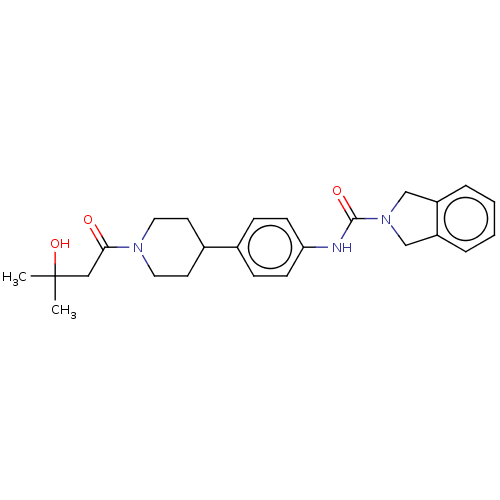 (CHEMBL4082366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50254184 (CHEMBL4082366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254184 (CHEMBL4082366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||