

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50254387 CHEMBL4062193
SMILES: Fc1ccc(Nc2n[nH]c3cc(OC(F)(F)F)cnc23)cc1F
InChI Key: InChIKey=QGIXOJCLJSPCPO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50254387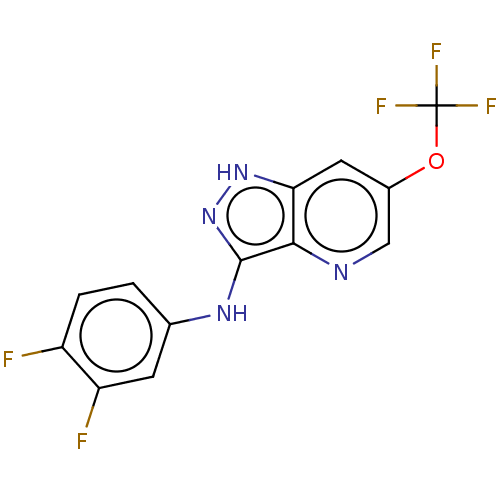 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of Nav1.5 (unknown origin) | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of Nav1.7 (unknown origin) | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of TRPV1 (unknown origin) | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional 3'-phosphoadenosine 5'-phosphosulfate synthase 2 (Homo sapiens) | BDBM50254387 (CHEMBL4062193) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Binding affinity to SK2 (unknown origin) | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Antagonist activity at human TRPA1 by calcium flux based FLIPR assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Antagonist activity at human TRPA1 by calcium flux based FLIPR assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KCNQ (Kv7) potassium channel (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of KCNQ1 (unknown origin) | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-gated potassium channel subunit Kv7.2/Kv7.3 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Activation of KCNQ2/3 (unknown origin) | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of full length human TRPA1 oS5 chimera at a holding potential of 15 mV measured after 1 min by PatchXpress electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of rat TRPA1 at a holding potential of 15 mV measured after 1 min by whole-cell manual patch clamp electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50254387 (CHEMBL4062193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of human ERG | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Rattus norvegicus) | BDBM50254387 (CHEMBL4062193) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire CB21 6GS, U.K. Curated by ChEMBL | Assay Description Inhibition of full length rat TRPA1 at a holding potential of 15 mV measured after 1 min by PatchXpress electrophysiology assay | ACS Med Chem Lett 8: 666-671 (2017) Article DOI: 10.1021/acsmedchemlett.7b00140 BindingDB Entry DOI: 10.7270/Q2C24ZWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||