

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50254647 2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy)-N-(4,5-dichlorothiophen-2-ylsulfonyl)acetamide::CHEMBL480809
SMILES: Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(c12)S(=O)(=O)c1ccc(Cl)c(Cl)c1
InChI Key: InChIKey=XUCAOVCVTZNGMX-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50254647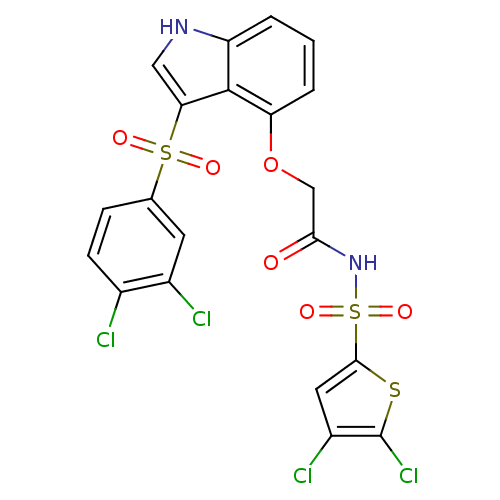 (2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor | Bioorg Med Chem Lett 19: 123-6 (2008) Article DOI: 10.1016/j.bmcl.2008.11.007 BindingDB Entry DOI: 10.7270/Q2JH3M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50254647 (2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor assessed as cAMP production by cell-based assay | Bioorg Med Chem Lett 19: 123-6 (2008) Article DOI: 10.1016/j.bmcl.2008.11.007 BindingDB Entry DOI: 10.7270/Q2JH3M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50254647 (2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor | Bioorg Med Chem Lett 19: 123-6 (2008) Article DOI: 10.1016/j.bmcl.2008.11.007 BindingDB Entry DOI: 10.7270/Q2JH3M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50254647 (2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum | Bioorg Med Chem Lett 19: 123-6 (2008) Article DOI: 10.1016/j.bmcl.2008.11.007 BindingDB Entry DOI: 10.7270/Q2JH3M1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||