

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@H]1NCCC[C@@H]1Nc1ncc(C(N)=O)c2sc(cc12)-c1ccccc1
InChI Key: InChIKey=LMQOZGSVTQQPFU-WBMJQRKESA-N
PDB links: 1 PDB ID matches this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50254814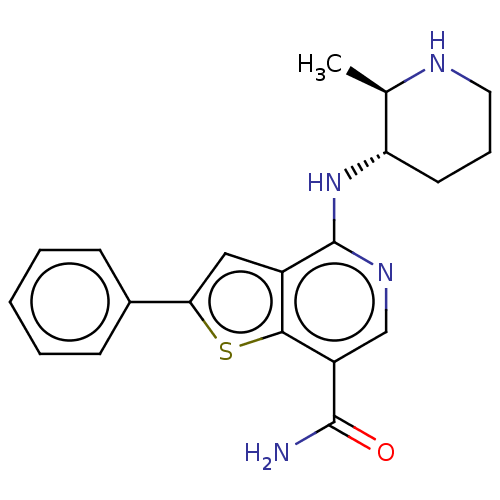 (CHEMBL4059912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Oncology Chemistry, IMED Biotech Unit, AstraZeneca , 35 Gatehouse Drive, Waltham, Massachusetts 02451, United States. Curated by ChEMBL | Assay Description Inhibition of CHK1 in human HT29 cells assessed as abrogation of camptothecin-induced G2/M phase arrest | J Med Chem 61: 1061-1073 (2018) Article DOI: 10.1021/acs.jmedchem.7b01490 BindingDB Entry DOI: 10.7270/Q2PC34T5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50254814 (CHEMBL4059912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Oncology Chemistry, IMED Biotech Unit, AstraZeneca , 35 Gatehouse Drive, Waltham, Massachusetts 02451, United States. Curated by ChEMBL | Assay Description Inhibition of CHK1 (unknown origin) | J Med Chem 61: 1061-1073 (2018) Article DOI: 10.1021/acs.jmedchem.7b01490 BindingDB Entry DOI: 10.7270/Q2PC34T5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50254814 (CHEMBL4059912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Oncology Chemistry, IMED Biotech Unit, AstraZeneca , 35 Gatehouse Drive, Waltham, Massachusetts 02451, United States. Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells at -80 mV holding potential by patch clamp assay | J Med Chem 61: 1061-1073 (2018) Article DOI: 10.1021/acs.jmedchem.7b01490 BindingDB Entry DOI: 10.7270/Q2PC34T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||