

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50255431 4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino)pentyl)cyclohexyl(methyl)carbamate 2-hydroxypropane-1,2,3-tricarboxylate::CHEMBL448434
SMILES: CN(CCCCC[C@H]1CC[C@@H](CC1)N(C)C(=O)Oc1ccc(cc1)C(F)(F)F)CC=C
InChI Key: InChIKey=QTPDFYJYCCDNKD-XUTJKUGGSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lanosterol synthase (Saccharomyces cerevisiae) | BDBM50255431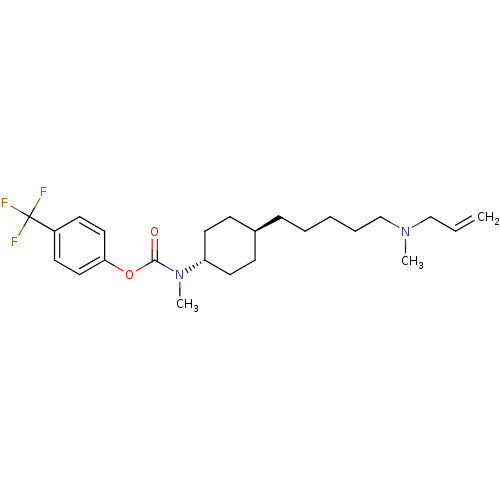 (4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino Curated by ChEMBL | Assay Description Inhibition of yeast OSC expressed in Saccharomyces cerevisiae SMY8 | Bioorg Med Chem Lett 19: 718-23 (2009) Article DOI: 10.1016/j.bmcl.2008.12.040 BindingDB Entry DOI: 10.7270/Q2SF2W2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50255431 (4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Inhibition of human liver microsome 2,3-OSC after 1 hr by Silica gel plate phosphor imaging | J Med Chem 55: 4990-5002 (2012) Article DOI: 10.1021/jm300256z BindingDB Entry DOI: 10.7270/Q2BZ674B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cycloartenol synthase (Arabidopsis thaliana) | BDBM50255431 (4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana cycloartenol synthase expressed in Saccharomyces cerevisiae SMY8 | Bioorg Med Chem Lett 19: 718-23 (2009) Article DOI: 10.1016/j.bmcl.2008.12.040 BindingDB Entry DOI: 10.7270/Q2SF2W2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50255431 (4-(trifluoromethyl)phenyl 4-(5-(allyl(methyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Torino Curated by ChEMBL | Assay Description Inhibition of human OSC expressed in Saccharomyces cerevisiae SMY8 | Bioorg Med Chem Lett 19: 718-23 (2009) Article DOI: 10.1016/j.bmcl.2008.12.040 BindingDB Entry DOI: 10.7270/Q2SF2W2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||