

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50255529 Ac-Lys(CoA)-NH2::CHEMBL505121
SMILES: CC(=O)N[C@@H](CCCCNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)n1cnc2c(N)ncnc12)C(N)=O
InChI Key: InChIKey=YGZKIOPJGFQDSR-QGSWMBNESA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone acetyltransferase PCAF (Homo sapiens (Human)) | BDBM50255529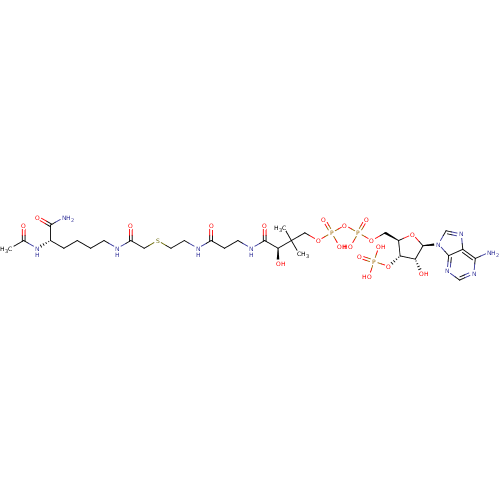 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PCAF expressed in baculovirus expression system using histone substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged p300 expressed in baculovirus expression system using histone substrate incubated for 10 mins... | J Med Chem 59: 1249-70 (2016) BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of recombinant full length p300 (unknown origin) using N-terminal H4-20 peptide substrate incubated for 7 mins by radiometric gel assay in... | J Med Chem 59: 1249-70 (2016) BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal GST-tagged p300 (1195 to 1673 residues) expressed in competent Escherichia coli DH5alpha cells using histo... | J Med Chem 59: 1249-70 (2016) BindingDB Entry DOI: 10.7270/Q2DV1MRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIF1A/p300/CREB-binding protein (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Avera Institute for Human Genetics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant p300 catalytic domain (1284-1673 residues) using histone H4-8 peptide and [14C]acetyl CoA as su... | J Med Chem 63: 4716-4731 (2020) Article DOI: 10.1021/acs.jmedchem.9b02164 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of recombinant p300 | Bioorg Med Chem 17: 1381-6 (2009) Article DOI: 10.1016/j.bmc.2008.12.014 BindingDB Entry DOI: 10.7270/Q2K35VKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase PCAF (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of PCAF HAT domain (493-658) expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem 17: 1381-6 (2009) Article DOI: 10.1016/j.bmc.2008.12.014 BindingDB Entry DOI: 10.7270/Q2K35VKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIF1A/p300/CREB-binding protein (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant FLAG-tagged human P300 expressed in Baculovirus expression system using histone peptide as substrate after 10 mins in prese... | Bioorg Med Chem 26: 5397-5407 (2018) Article DOI: 10.1016/j.bmc.2018.07.048 BindingDB Entry DOI: 10.7270/Q2ZW1PM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIF1A/p300/CREB-binding protein (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human p300 using [14C]acetyl-CoA as substrate measured after 10 mins by phosphor imaging analysis | J Med Chem 63: 1337-1360 (2020) Article DOI: 10.1021/acs.jmedchem.9b01721 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT5 (Homo sapiens (Human)) | BDBM50255529 (Ac-Lys(CoA)-NH2 | CHEMBL505121) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of recombinant Tip60 (1-513) expressed in Escherichia coli BL21 (DE3) by liquid scintillation | Bioorg Med Chem 17: 1381-6 (2009) Article DOI: 10.1016/j.bmc.2008.12.014 BindingDB Entry DOI: 10.7270/Q2K35VKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||