

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50256791 CHEMBL4090412
SMILES: [H][C@@]12[#6][C@@]3([H])[#6@H](-[#8]-[#6])-[#6]4-[#6](=O)-c5c(-[#8])c6-[#6]=[#6]C([#6])([#6])[#8]-c6c(-[#6]\[#6]=[#6](\[#6])-[#6])c5-[#8][C@@]14[C@@]([#6]\[#6]=[#6](\[#6])-[#6](-[#8])=O)([#8]C2([#6])[#6])[#6]3=O
InChI Key: InChIKey=YWPUDSYPVZVGNW-FVFQGOTJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50256791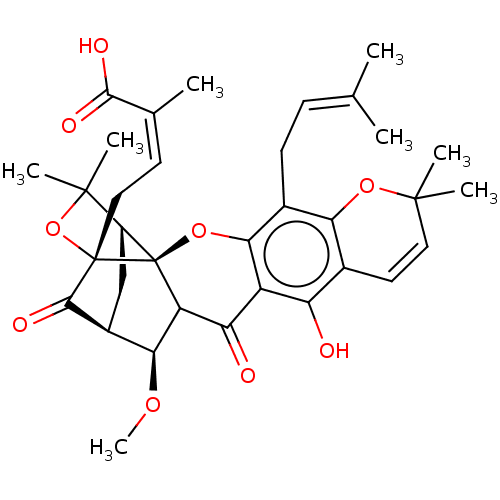 (CHEMBL4090412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate by Dixon plot analysis | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50256791 (CHEMBL4090412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Applied Life Science (BK21 Plus), IALS, Gyeongsang National University, Jinju 52828, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B (1 to 322 residues) expressed in Escherichia coli using pNPP as substrate incubated for 10 mins measured for 30... | Bioorg Med Chem 25: 2498-2506 (2017) Article DOI: 10.1016/j.bmc.2017.03.010 BindingDB Entry DOI: 10.7270/Q2Q81GHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||