

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50257503 CHEMBL4067877
SMILES: O=C(NCc1ccccc1)c1cccc(NCc2nc(c[nH]2)-c2ccncc2)c1
InChI Key: InChIKey=BBZVXUBFCDUWHJ-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50257503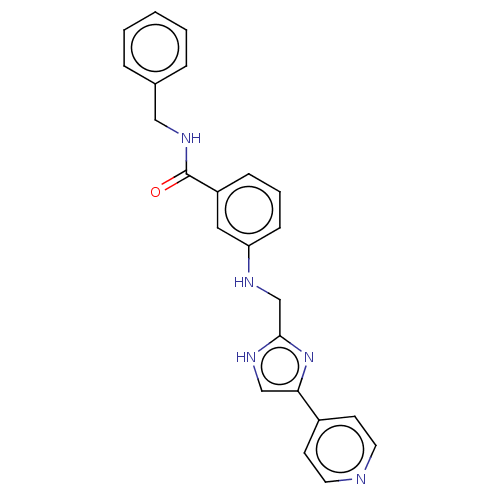 (CHEMBL4067877) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged ROCK2 catalytic domain (1 to 553 residues) expressed in baculovirus expression system using STK... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PKC alpha and beta-2 (Homo sapiens (Human)) | BDBM50257503 (CHEMBL4067877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human PKCalpha active using MBP as substrate after 60 mins in presence of [gamma-32]ATP by scintillation counting | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50257503 (CHEMBL4067877) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of human GRK2 expressed in HEK-B2 cells assessed as isoproterenol-stimulated cAMP accumulation preincubation for 20 mins followed by isopr... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||