

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50258636 1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carboxamido)-2,4,6-trimethylpyridinium perchlorate::1-({[5-(Aminosulfonyl)-3-(2-fluorophenyl)-1H-indol-2-yl]carbonyl}amino)-2,4,6 trimethylpyridinium perchlorate::CHEMBL513835
SMILES: Cc1cc(C)[n+](NC(=O)c2[nH]c3ccc(cc3c2-c2ccccc2Cl)S(N)(=O)=O)c(C)c1
InChI Key: InChIKey=TXDBKBXULDSLMV-UHFFFAOYSA-O
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50258636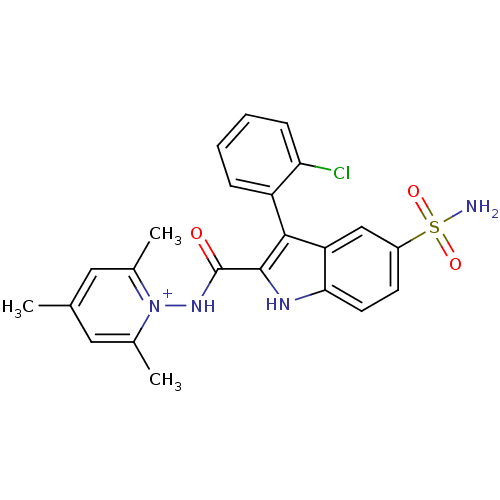 (1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase (mtCA 1) (Mycobacterium tuberculosis) | BDBM50258636 (1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv1284 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Carbonic Anhydrase (Candida albicans (Yeast)) | BDBM50258636 (1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description inhibition of Candida albicans recombinant Nce103 after 15 mins by stopped-flow CO2 hydration assay | Bioorg Med Chem Lett 20: 2508-11 (2010) Article DOI: 10.1016/j.bmcl.2010.02.103 BindingDB Entry DOI: 10.7270/Q23F4PS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258636 (1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of human recombinant CA1 by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PROBABLE TRANSMEMBRANE CARBONIC ANHYDRASE (CARBONATE DEHYDRATASE) (CARBONIC DEHYDRATASE) (Mycobacterium tuberculosis) | BDBM50258636 (1-(3-(2-chlorophenyl)-5-sulfamoyl-1H-indole-2-carb...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istanbul University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant carbonic anhydrase Rv3273 by by stopped flow CO2 hydration assay | J Med Chem 52: 4063-7 (2009) Article DOI: 10.1021/jm9004016 BindingDB Entry DOI: 10.7270/Q2736QTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||