

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50259954 CHEMBL4094377
SMILES: CC(C)(NCc1cc(c(F)cc1F)[C@]1(C)CCSC(N)=N1)C(F)(F)F
InChI Key: InChIKey=JMOHXAUCSPMQGB-HNNXBMFYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM50259954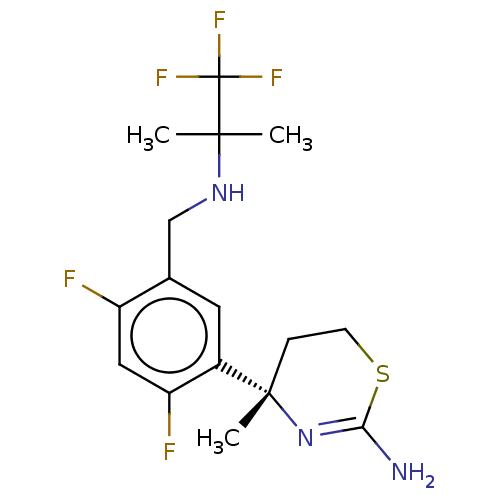 (CHEMBL4094377) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Cathepsin D (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259954 (CHEMBL4094377) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259954 (CHEMBL4094377) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass... | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50259954 (CHEMBL4094377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells at -80 mV holding potential after 5 mins by patch clamp assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50259954 (CHEMBL4094377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) by cell free assay | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||