 Found 17 hits for monomerid = 50265078
Found 17 hits for monomerid = 50265078 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-12 (MMP12)
(Homo sapiens (Human)) | BDBM50265078
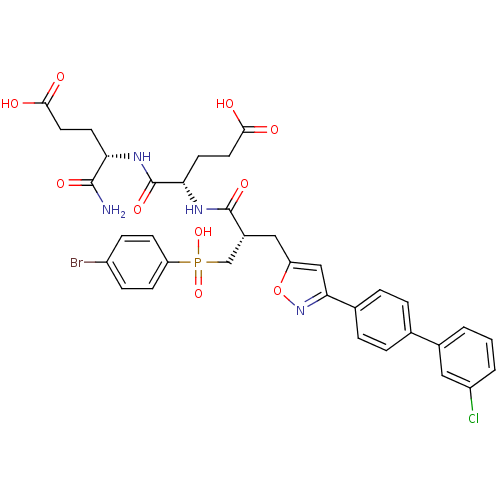
((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP12 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-12 (MMP12)
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
CEA
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 catalytic domain (98 to 266) at pH 6.8 by isothermal titration calorimetry |
J Med Chem 56: 1149-59 (2013)
Article DOI: 10.1021/jm301574d
BindingDB Entry DOI: 10.7270/Q24F1S24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-12 (MMP12)
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA
Curated by ChEMBL
| Assay Description
Binding affinity to human MMP12 catalytic domain (98 to 266) by isothermal titration calorimetry |
J Med Chem 56: 1149-59 (2013)
Article DOI: 10.1021/jm301574d
BindingDB Entry DOI: 10.7270/Q24F1S24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate by spectrophotometric analysis |
J Med Chem 60: 403-414 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01420
BindingDB Entry DOI: 10.7270/Q24X5B7B |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14 (MMP14)
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase 10
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay
Curated by ChEMBL
| Assay Description
Inhibition of human MMP10 catalytic domain using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate by spectrophotometric analysis |
J Med Chem 60: 403-414 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01420
BindingDB Entry DOI: 10.7270/Q24X5B7B |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP8 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-7 (MMP7)
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase 11
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP11 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50265078

((4S)-5-amino-4-((2S)-2-((2S)-3-((4-bromophenyl)(hy...)Show SMILES NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cc(no1)-c1ccc(cc1)-c1cccc(Cl)c1)CP(O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C35H35BrClN4O10P/c36-24-8-10-27(11-9-24)52(49,50)19-23(34(47)40-29(13-15-32(44)45)35(48)39-28(33(38)46)12-14-31(42)43)17-26-18-30(41-51-26)21-6-4-20(5-7-21)22-2-1-3-25(37)16-22/h1-11,16,18,23,28-29H,12-15,17,19H2,(H2,38,46)(H,39,48)(H,40,47)(H,42,43)(H,44,45)(H,49,50)/t23-,28+,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of NEP |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data