

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50265674 CHEMBL3734774
SMILES: Fc1ccc(cc1)-n1ncc2C[C@@]3(CN(CCC3=Cc12)S(=O)(=O)c1ccc(cc1)C(F)(F)F)C(=O)c1ccccn1
InChI Key: InChIKey=VXOBXKQLNWYQPQ-NDEPHWFRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674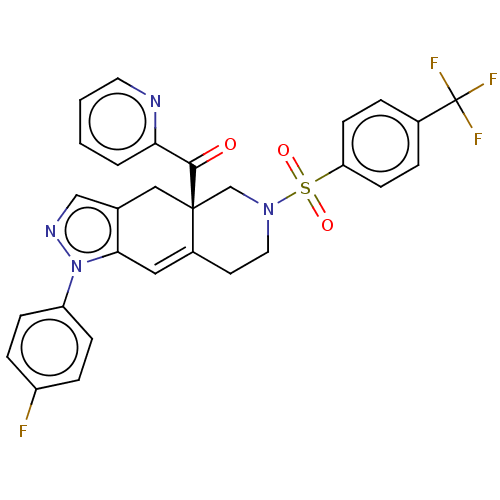 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Binding affinity to human recombinant glucocorticoid receptor by fluorescence polarization assay | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of fluormone GS Red from human glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of dexamethasone-induced tyrosine amino transferase activi... | J Med Chem 60: 3405-3421 (2017) Article DOI: 10.1021/acs.jmedchem.7b00162 BindingDB Entry DOI: 10.7270/Q27083X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50265674 (CHEMBL3734774) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in rat primary hepatocytes assessed as inhibition of glucocorticoid-induced TAT activity | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human HepG2 cells assessed as inhibition of glucocorticoid-induced TAT activity after 24 hrs | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50265674 (CHEMBL3734774) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at glucocorticoid receptor in human primary hepatocytes assessed as inhibition of glucocorticoid-induced TAT activity | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50265674 (CHEMBL3734774) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in rat primary hepatocytes assessed as inhibition of glucocorticoid-induced TAT activity | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50265674 (CHEMBL3734774) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Inhibition of human ERG by patch clamp technique | Bioorg Med Chem Lett 25: 5720-5 (2015) Article DOI: 10.1016/j.bmcl.2015.10.097 BindingDB Entry DOI: 10.7270/Q2ST7STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||