

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: FC(F)(F)c1ccc(CC(=O)N[C@H]2CCOC2=O)cc1
InChI Key: InChIKey=HYPQMBQCQQDGGJ-JTQLQIEISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcriptional activator protein TraR (Rhizobium radiobacter) | BDBM50266165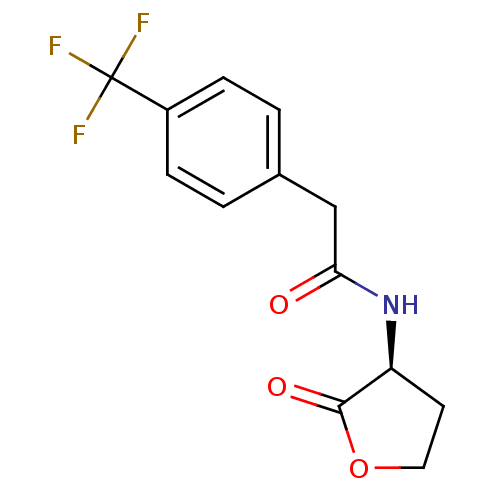 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Antagonist activity at Agrobacterium tumefaciens WCF47 TraR receptor assessed as inhibition of OOHL-induced response by beta-galctosidase reporter ge... | Bioorg Med Chem Lett 18: 5978-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.089 BindingDB Entry DOI: 10.7270/Q2Q81CXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein LuxR (Vibrio fischeri (strain ATCC 700601 / ES114)) | BDBM50266165 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Antagonist activity at Vibrio fischeri ES114 LuxR receptor assessed as inhibition of OHHL-induced response by luciferase reporter gene assay | Bioorg Med Chem Lett 18: 5978-81 (2008) Article DOI: 10.1016/j.bmcl.2008.07.089 BindingDB Entry DOI: 10.7270/Q2Q81CXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein LuxR (Vibrio fischeri) | BDBM50266165 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Vibrio fischeri luxR receptor in presence of 5 uM 3-oxo-C6-HSL | J Med Chem 53: 7467-89 (2010) Article DOI: 10.1021/jm901742e BindingDB Entry DOI: 10.7270/Q2S75GPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein LuxR (Vibrio fischeri) | BDBM50266165 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Larkin University Curated by ChEMBL | Assay Description Antagonist activity at Aliivibrio fischeri ES114 LuxR after 4 to 8 hrs by luminescence method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115282 BindingDB Entry DOI: 10.7270/Q2PN992D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein LasR (Pseudomonas aeruginosa) | BDBM50266165 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Larkin University Curated by ChEMBL | Assay Description Inhibition of LasR in Pseudomonas aeruginosa PAO-JP2 harboring plasI-LVAgfp incubated for 6 hrs by fluorescence method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115282 BindingDB Entry DOI: 10.7270/Q2PN992D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein LasR (Pseudomonas aeruginosa) | BDBM50266165 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a |
Larkin University Curated by ChEMBL | Assay Description Activation of LasR in Pseudomonas aeruginosa PAO-JP2 harboring plasI-LVAgfp incubated for 6 hrs by fluorescence method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115282 BindingDB Entry DOI: 10.7270/Q2PN992D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein TraR (Rhizobium radiobacter) | BDBM50266165 ((S)-N-(2-oxotetrahydrofuran-3-yl)-2-(4-(trifluorom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at Agrobacterium tumefaciens TraR receptor in presence of 100 nM 3-oxo-C8-HSL | J Med Chem 53: 7467-89 (2010) Article DOI: 10.1021/jm901742e BindingDB Entry DOI: 10.7270/Q2S75GPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||