

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50267874 (4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)phenylsulfonyl)piperazin-1-yl)((1R,2R)-2-(4-(trifluoromethyl)phenyl)cyclopropyl)methanone::CHEMBL528997
SMILES: FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1c[nH]nn1
InChI Key: InChIKey=KHIVVJPXJZJALG-VQTJNVASSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267874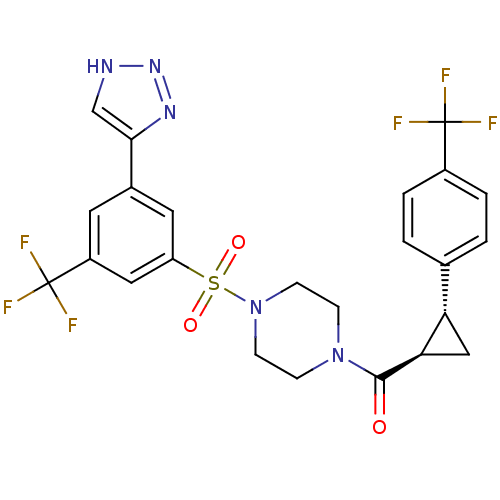 ((4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267874 ((4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267874 ((4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||