

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50268687 CHEMBL522725::sodium 1-amino-4-(2-hydroxyphenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate
SMILES: Nc1c(cc(Nc2ccccc2O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O
InChI Key: InChIKey=PPOIMBFKMLPDFQ-UHFFFAOYSA-M
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecto-5'-nucleotidase (e5'NT) (Rattus norvegicus (Rat)) | BDBM50268687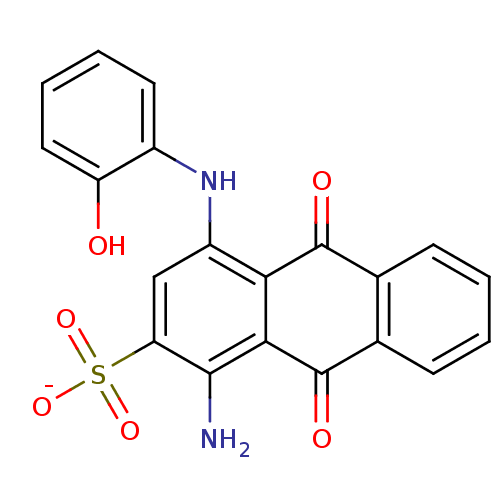 (CHEMBL522725 | sodium 1-amino-4-(2-hydroxyphenylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y12 (Homo sapiens (Human)) | BDBM50268687 (CHEMBL522725 | sodium 1-amino-4-(2-hydroxyphenylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB0413 from human platelet P2Y12 receptor | J Med Chem 52: 3784-93 (2009) Article DOI: 10.1021/jm9003297 BindingDB Entry DOI: 10.7270/Q2NK3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 4 (RAT) | BDBM50268687 (CHEMBL522725 | sodium 1-amino-4-(2-hydroxyphenylam...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity against rat P2X4 receptor expressed in Xenopus laevis oocyte assessed as inhibition of alpha, beta-meATP-induced inward current b... | J Med Chem 54: 817-30 (2012) Article DOI: 10.1021/jm1012193 BindingDB Entry DOI: 10.7270/Q2VH5PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic, P2X2 (RAT) | BDBM50268687 (CHEMBL522725 | sodium 1-amino-4-(2-hydroxyphenylam...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity against rat P2X2 receptor expressed in Xenopus laevis oocyte assessed as inhibition of alpha, beta-meATP-induced inward current b... | J Med Chem 54: 817-30 (2012) Article DOI: 10.1021/jm1012193 BindingDB Entry DOI: 10.7270/Q2VH5PTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||