

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50274182 CHEMBL484392::N-Formyl-L-proline-(1S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]dodecyl Ester
SMILES: CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@@H]1CCCN1C=O
InChI Key: InChIKey=GRWXJSVBCXVHND-CQJMVLFOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50274182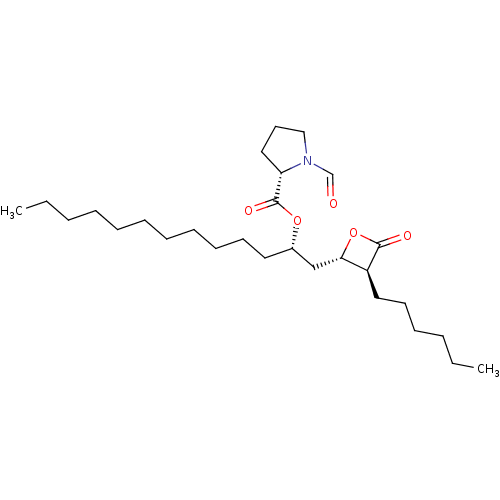 (CHEMBL484392 | N-Formyl-L-proline-(1S)-1-[[(2S,3S)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in HEK293 cells by scintillation counting | J Med Chem 51: 6970-9 (2008) Article DOI: 10.1021/jm800978m BindingDB Entry DOI: 10.7270/Q2251J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50274182 (CHEMBL484392 | N-Formyl-L-proline-(1S)-1-[[(2S,3S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2 receptor expressed in HEK293 cells by scintillation counting | J Med Chem 51: 6970-9 (2008) Article DOI: 10.1021/jm800978m BindingDB Entry DOI: 10.7270/Q2251J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (aa 30-579) (Rattus norvegicus (rat)) | BDBM50274182 (CHEMBL484392 | N-Formyl-L-proline-(1S)-1-[[(2S,3S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma Curated by ChEMBL | Assay Description Inhibition of FAAH-mediated [14C]anadamide hydrolysis in rat brain membrane | J Med Chem 51: 6970-9 (2008) Article DOI: 10.1021/jm800978m BindingDB Entry DOI: 10.7270/Q2251J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sn1-specific diacylglycerol lipase alpha (Homo sapiens (Human)) | BDBM50274182 (CHEMBL484392 | N-Formyl-L-proline-(1S)-1-[[(2S,3S)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Università di Roma Curated by ChEMBL | Assay Description Inhibition of human recombinant DAGLalpha-mediated sn-1-[14C]oleoyl-2-arachidonoyl-glycerol hydrolysis to 2-AG overexpressed in african green monkey ... | J Med Chem 51: 6970-9 (2008) Article DOI: 10.1021/jm800978m BindingDB Entry DOI: 10.7270/Q2251J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||