

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50274257 (2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-10,17-dihydroxy-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7,9,11(18)-trien-14-yl]-3-(furan-2-yl)-N-methylprop-2-enamide::(2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxymorphinan-6-yl]-3-(furan-2-yl)-N-methylprop-2-enamide::CHEMBL485605::TRK-820
SMILES: CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccco1
InChI Key: InChIKey=VJGYEDILSGGSMK-NUINQRJCSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50274257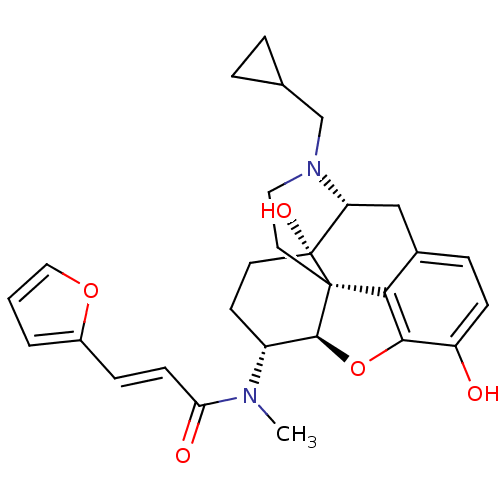 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to kappa opioid receptor | Bioorg Med Chem 18: 4446-52 (2010) Article DOI: 10.1016/j.bmc.2010.04.069 BindingDB Entry DOI: 10.7270/Q23X87MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50274257 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from guinea pig cerebellum kappa opioid receptor | Bioorg Med Chem Lett 20: 121-4 (2010) Article DOI: 10.1016/j.bmcl.2009.11.027 BindingDB Entry DOI: 10.7270/Q2GX4CJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50274257 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.582 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from guinea pig forebrain mu opioid receptor | Bioorg Med Chem Lett 20: 121-4 (2010) Article DOI: 10.1016/j.bmcl.2009.11.027 BindingDB Entry DOI: 10.7270/Q2GX4CJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 1 (Homo sapiens (Human)) | BDBM50274257 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 751 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50274257 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in ddy mouse vas deferens assessed as inhibition of electric stimulation-induced contraction | Bioorg Med Chem 16: 9188-201 (2008) Article DOI: 10.1016/j.bmc.2008.09.011 BindingDB Entry DOI: 10.7270/Q2736RVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||