

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50274303 CHEMBL502862::p-Methoxyphenyl (3,5,9-trideoxy-5-glycolamido-9-(4-hydroxy-4-biphenyl)-methylamino-D-glycero-alpha-D-galacto-2-nonulopyranosylonicacid)-(2->6)-beta-D-galactopyranoside
SMILES: COc1ccc(O[C@@H]2O[C@H](CO[C@@]3(C[C@H](O)[C@@H](NC(=O)CO)[C@@H](O3)[C@H](O)[C@H](O)CNCc3ccc(cc3)-c3ccc(O)cc3)C(O)=O)[C@H](O)[C@H](O)[C@H]2O)cc1
InChI Key: InChIKey=QATDWUKLFZZIKB-GFYPMFDPSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD22 (Homo sapiens (Human)) | BDBM50274303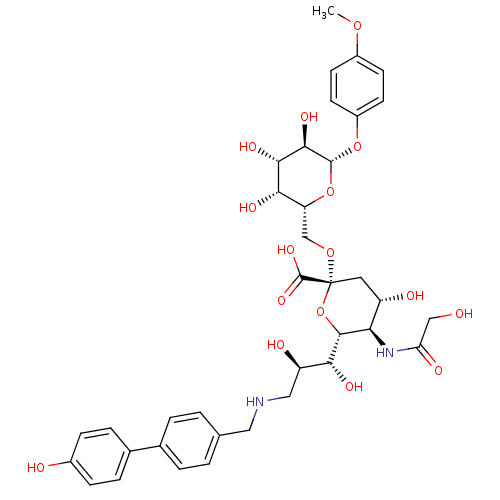 (CHEMBL502862 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Binding affinity to biotinylated human CD22-human IgG1 chimeric protein expressed in mouse J558LST6 cells by flow cytometry | J Med Chem 51: 6665-81 (2008) Article DOI: 10.1021/jm8000696 BindingDB Entry DOI: 10.7270/Q23X86GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myelin-associated glycoprotein (Homo sapiens (Human)) | BDBM50274303 (CHEMBL502862 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human MAG after 1 hr by competitive ELISA | Bioorg Med Chem 19: 1966-71 (2011) Article DOI: 10.1016/j.bmc.2011.01.060 BindingDB Entry DOI: 10.7270/Q2QZ2B7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CD22 (Homo sapiens (Human)) | BDBM50274303 (CHEMBL502862 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...) | PDB NCI pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Antagonist activity at human CD22 after 1 hr by competitive ELISA | Bioorg Med Chem 19: 1966-71 (2011) Article DOI: 10.1016/j.bmc.2011.01.060 BindingDB Entry DOI: 10.7270/Q2QZ2B7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell receptor CD22 (Mus musculus) | BDBM50274303 (CHEMBL502862 | p-Methoxyphenyl (3,5,9-trideoxy-5-g...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Antagonist activity at mouse CD22 after 1 hr by competitive ELISA | Bioorg Med Chem 19: 1966-71 (2011) Article DOI: 10.1016/j.bmc.2011.01.060 BindingDB Entry DOI: 10.7270/Q2QZ2B7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||