

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50276437 (2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-7-(pyridin-4-ylmethoxy)-1,2,3,4,4a,9,10,10a-octahydrophenanthren-2-ol::CHEMBL457035
SMILES: CC[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(OCc3ccncc3)ccc21)C#CC
InChI Key: InChIKey=GUJAUKQZCIPIBV-NQHRYMMQSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50276437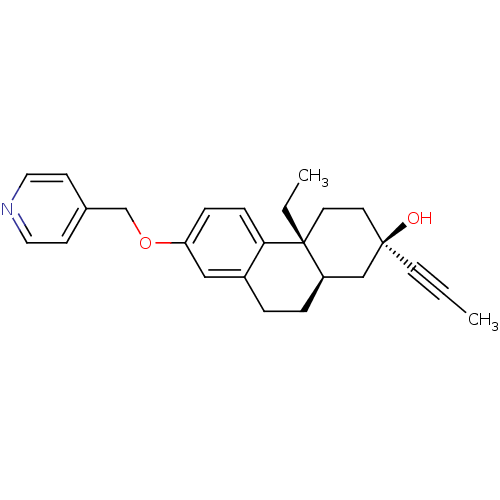 ((2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-7-(pyridin-4-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human ERbeta | J Med Chem 52: 1731-43 (2009) Article DOI: 10.1021/jm801512v BindingDB Entry DOI: 10.7270/Q23778M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50276437 ((2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-7-(pyridin-4-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Transrepression activity at GR in human SW1353 cells assessed as inhibition of IL1-stimulated MMP13 production after 24 hrs by whole cell ELISA | J Med Chem 52: 1731-43 (2009) Article DOI: 10.1021/jm801512v BindingDB Entry DOI: 10.7270/Q23778M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50276437 ((2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-7-(pyridin-4-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human glucocorticoid receptor | J Med Chem 52: 1731-43 (2009) Article DOI: 10.1021/jm801512v BindingDB Entry DOI: 10.7270/Q23778M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50276437 ((2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-7-(pyridin-4-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human ERalpha | J Med Chem 52: 1731-43 (2009) Article DOI: 10.1021/jm801512v BindingDB Entry DOI: 10.7270/Q23778M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||