

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50276554 (1S,2R)-N-{1-Benzyl-2-hydroxy-3-(S)-[2-(1-benzylpiperidin-4-yl)ethylamino]-propyl}-5-[methyl(methylsulfonyl)amino]-N'-[(R)-1-phenylethyl]isophthalamide::CHEMBL510269
SMILES: C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCCC1CCN(Cc2ccccc2)CC1)N(C)S(C)(=O)=O)c1ccccc1
InChI Key: InChIKey=IMNAYTZSJCRZIO-RLRCJDGOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50276554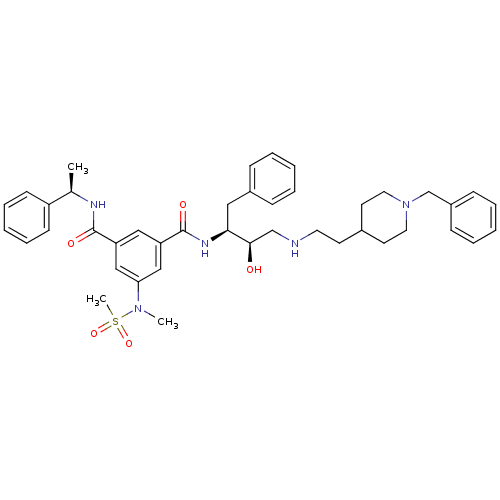 ((1S,2R)-N-{1-Benzyl-2-hydroxy-3-(S)-[2-(1-benzylpi...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 in HEK293 cells transfected with human betaAPP695 mutant assessed as inhibition of amyloid beta (1 to 40) production after 24 hrs... | Bioorg Med Chem 17: 1600-13 (2009) Article DOI: 10.1016/j.bmc.2008.12.067 BindingDB Entry DOI: 10.7270/Q24J0F0R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50276554 ((1S,2R)-N-{1-Benzyl-2-hydroxy-3-(S)-[2-(1-benzylpi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of AChE in rat cortex preincubated for 20 mins by Ellman method | Bioorg Med Chem 17: 1600-13 (2009) Article DOI: 10.1016/j.bmc.2008.12.067 BindingDB Entry DOI: 10.7270/Q24J0F0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50276554 ((1S,2R)-N-{1-Benzyl-2-hydroxy-3-(S)-[2-(1-benzylpi...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 567 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal histidine-tagged BACE1 expressed in baculovirus-infected insect cells by FRET assay | Bioorg Med Chem 17: 1600-13 (2009) Article DOI: 10.1016/j.bmc.2008.12.067 BindingDB Entry DOI: 10.7270/Q24J0F0R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||