

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50280623 5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine::9-Amino-5-chloro-1,2,3,4-tetrahydro-acridinium::CHEMBL369661
SMILES: Nc1c2CCCCc2nc2c(Cl)cccc12
InChI Key: InChIKey=DREAHDLCWZKIJL-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50280623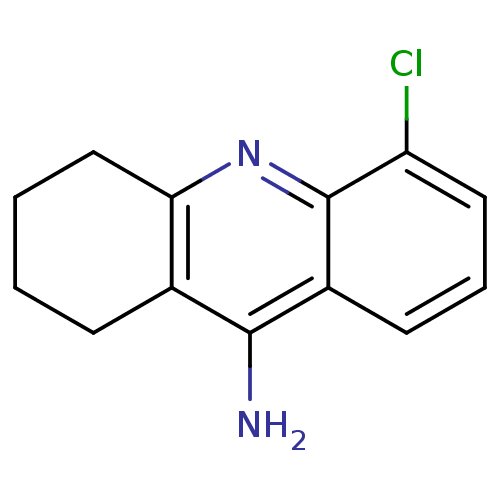 (5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of (BChE) Butyrylcholinesterase of horse serum | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50280623 (5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description In vitro inhibitory activity against human erythrocyte acetylcholinesterase | J Med Chem 47: 4471-82 (2004) Article DOI: 10.1021/jm049877p BindingDB Entry DOI: 10.7270/Q26974WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50280623 (5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) of human red blood cell (type XIII) by modified radiometric AChE assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50280623 (5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in electric eel (type V-S) by modified radiometric assay | Bioorg Med Chem Lett 2: 861-864 (1992) Article DOI: 10.1016/S0960-894X(00)80545-4 BindingDB Entry DOI: 10.7270/Q29886XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||