

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50280829 2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1-yl]-ethyl}-1,1-dioxo-2,3-dihydro-1H-1lambda*6*-benzo[e][1,2]thiazin-4-one::CHEMBL57198
SMILES: OC1=CN(CCN2CCC(Cc3c[nH]c4ccc(F)cc34)CC2)S(=O)(=O)c2ccccc12
InChI Key: InChIKey=YELYMKQLVXIVMT-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50280829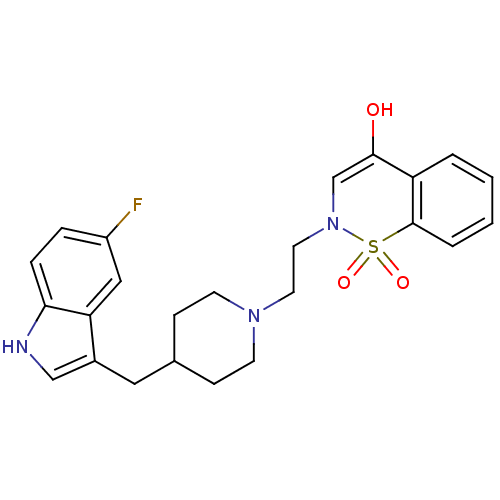 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Dopamine receptor D2; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor (Homo sapiens (Human)) | BDBM50280829 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1 adrenergic receptor (Mus musculus (Mouse)) | BDBM50280829 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its binding affinity against Alpha-1 adrenergic receptor; showed no appreciable affinity at concentration specified | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 1 (5-HT1) receptor (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280829 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||