

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50281782 (1S,4R)-2-{2-[2-[((S)-{(S)-1-[2-((S)-2-Amino-5-guanidino-pentanoylamino)-propionyl]-pyrrolidin-2-yl}-oxo-methyl)-amino]-3-((S)-4-hydroxy-phenyl)-propionylamino]-3-methyl-pentanoylamino}-4-methyl-pentanoic acid::CHEMBL172473
SMILES: CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O
InChI Key: InChIKey=SCMSMSCMAVVGFP-QJKSWXJZSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor 1 (MOUSE) | BDBM50281782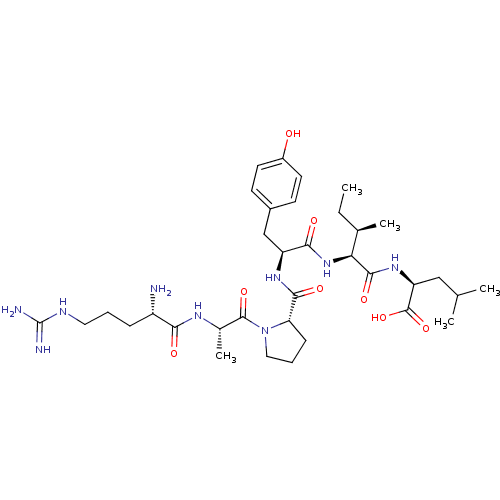 ((1S,4R)-2-{2-[2-[((S)-{(S)-1-[2-((S)-2-Amino-5-gua...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-Tyr3-NT(1-13) to NT receptors in neonatal mouse whole brain (minus cerebellum) | Bioorg Med Chem Lett 3: 949-952 (1993) Article DOI: 10.1016/S0960-894X(00)80698-8 BindingDB Entry DOI: 10.7270/Q2CJ8DDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||