

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50284797 7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxolo[4',5':4,5]benzo[1,2,3-de]quinoline::CHEMBL36654::GNF-Pf-4466::cid_235224
SMILES: CN1CCc2cc3OCOc3c3-c4ccccc4CC1c23
InChI Key: InChIKey=JCTYWRARKVGOBK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50284797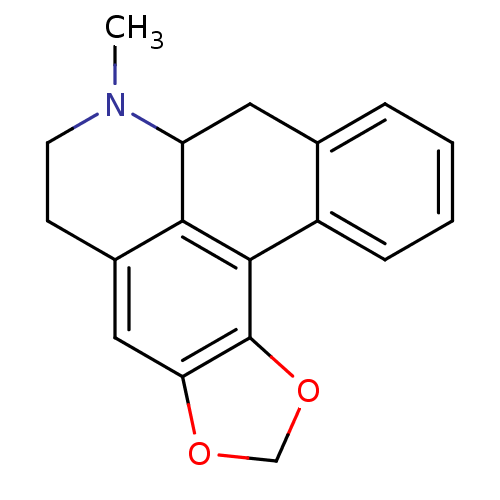 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from D2 receptor of Wistar rat striatal membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 23: 4824-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.078 BindingDB Entry DOI: 10.7270/Q2BK1G9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from D2 receptor of Wistar rat striatal membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 23: 4824-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.078 BindingDB Entry DOI: 10.7270/Q2BK1G9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from D1 receptor of Wistar rat striatal membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 23: 4824-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.078 BindingDB Entry DOI: 10.7270/Q2BK1G9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Valencia Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from D1 receptor of Wistar rat striatal membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem Lett 23: 4824-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.078 BindingDB Entry DOI: 10.7270/Q2BK1G9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2F18X8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant CD45 protein tyrosine phosphatase | Bioorg Med Chem Lett 5: 1519-1522 (1995) Article DOI: 10.1016/0960-894X(95)00250-W BindingDB Entry DOI: 10.7270/Q2V69K2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptotic peptidase activating factor 1 (Homo sapiens (Human)) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2JS9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-9 (Homo sapiens (Human)) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q25M647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RecName: Full=Zinc finger protein mex-5 (Caenorhabditis elegans) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Broad Institute: MLPCN maternal gene expression Project ID: 2024 Keywords: Zinc finger, C. elegans, maternal gene expression, RNA-protein interac... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2D798VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POsterior Segregation family member (pos-1) (Caenorhabditis elegans) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Broad Institute: MLPCN maternal gene expression Project ID: 2024 Keywords: Zinc finger, C. elegans, maternal gene expression, RNA-protein interac... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q28G8J4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methyl-CpG binding domain protein 2 (Homo sapiens (Human)) | BDBM50284797 (7-Methyl-6,7,7a,8-tetrahydro-5H-benzo[g][1,3]dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide... | PubChem Bioassay (2013) BindingDB Entry DOI: 10.7270/Q20K2778 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||