

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50289001 2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazin-4-one::CHEMBL349763::US9695194, 18
SMILES: COc1cc2nc(oc(=O)c2cc1OC)-c1ccccc1I
InChI Key: InChIKey=HKRJBRGRGFMQJI-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kallikrein 7 (Homo sapiens (Human)) | BDBM50289001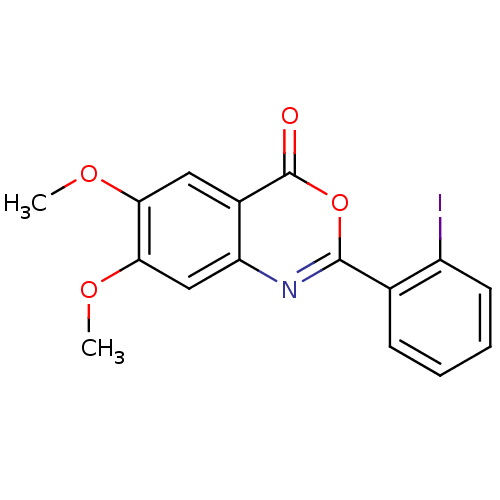 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 100 | -9.93 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Recombinant human KLK7, Substrate S-2586 (Chromogenics, cat. no. 820894) KLK7 activity was determined at 37° C. in 10 mM Na phosphate ... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.90E+3 | -8.11 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Cathepsin G, 100 mU (VWR, Calbiochem, cat. no. 219373), Substrate Cathepsin G substrate (VWR, Calbiochem, cat. no. 219407). Cathepsin acti... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein 14 (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.10E+3 | -8.05 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Recombinant human KLK14, Substrate S-2302 (Chromogenics, cat. no. 820340). KLK14 activity was determined at 37° C. in 0.1 mM Tris, pH ... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein 5 (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB MMDB KEGG B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.50E+3 | -7.58 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Recombinant human KLK5, Substrate S-2288 (Chromogenics, cat. no. 820852) KLK5 activity was determined at 37° C. in 0.1 M Tris, pH 8.0,... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | >1.00E+4 | >-7.09 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Chymotrypsin, bovine, 25 ug (Roche, sequence grade), Substrate S-2586 (Chromogenics, cat. no. 82 08 94). Chymotrypsin activity was determi... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | >1.00E+4 | >-7.09 | n/a | n/a | n/a | n/a | n/a | 8.3 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Thrombin (Chromogenics, cat. no. 820712), Substrate S-2288 (Chromogenics, cat. no. 82 08 52). Thrombin activity was determined at 37° ... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | >1.00E+4 | >-7.09 | n/a | n/a | n/a | n/a | n/a | 7.2 | 37 |
SIXERA PHARMA AB US Patent | Assay Description Materials: Trypsin, 100 ug (Roche, sequence grade, Mw 23500), Substrate S-2288 (Chromogenics, cat. no. 820852). Trypsin activity was determined at 3... | US Patent US9695194 (2017) BindingDB Entry DOI: 10.7270/Q2CN723D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r subcomponent (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement C1r (Homo sapiens (Human)) | BDBM50289001 (2-(2-Iodo-phenyl)-6,7-dimethoxy-benzo[d][1,3]oxazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >6.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of C1r serine protease | Bioorg Med Chem Lett 6: 679-682 (1996) Article DOI: 10.1016/0960-894X(96)00094-7 BindingDB Entry DOI: 10.7270/Q2P84BVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||